Questions
\(2FeO(s)\rightleftharpoons 2Fe(s)+O_{2} K_{eq}\)= \(1\times 10^{-6}\) at 1000K
\(CO_{2}(g)\rightleftharpoons C(s)+O_{2}(G) K_{eq}\)= \(1\times 10^{-32} \)at 1000K
The formation of Fe(s) and\( O_2\)(g) from FeO(s) is not thermodynamically favorable at room temperature. In an effort to make the process favorable, C(s) is added to the FeO(s) at elevated temperatures. Based on the information above, which of the following gives the value of K eq and the sign of \(\Delta G^{\circ}\) for the reaction represented by the equation below at 1000 K?
$2FeO(s)+ C_{s} \rightleftharpoons 2Fe(s)+CO_{2}$
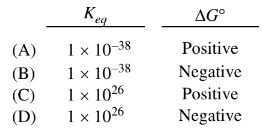
▶️Answer/Explanation
Ans: D
Given:
1. \(K_{eq}\) for \(2FeO(s) \rightleftharpoons 2Fe(s) + O_{2}\) is \(1 \times 10^{-6}\) at 1000 K.
2. \(K_{eq}\) for \(CO_{2}(g) \rightleftharpoons C(s) + O_{2}(g)\) is \(1 \times 10^{-32}\) at 1000 K.
To find the equilibrium constant \(K_{eq}\) for the overall reaction:
\[ 2FeO(s) + C_{s} \rightleftharpoons 2Fe(s) + CO_{2} \]
We need to consider the equilibrium constant for the individual reactions and apply them to determine the equilibrium constant for the overall reaction.
Given the equilibrium constants for the individual reactions, we know:
The reverse reactions are favored at 1000 K for both reactions because their equilibrium constants are very small.
This suggests that the forward reactions are thermodynamically unfavorable.
For the overall reaction, it involves the combination of two unfavorable forward reactions. Thus, the overall equilibrium constant will be extremely small, as it is the product of the equilibrium constants of the
individual reactions.
\[K_{eq}=K_{1} \times K_{2} = (1 times 10^{-6}) \times (1 \times 10^{32}) =1 \times 10^{26} \]
Given that the overall reaction proceeds in the thermodynamically unfavorable direction, the sign of \(AG^{\circ}\) for the overall reaction will be negative.
Therefore, the correct answer is:
(D) \(1 \times 10^{26}\) & Negative
Questions
\(2FeO(s)\rightleftharpoons 2Fe(s)+O_{2} K_{eq}\)= \(1\times 10^{-6}\) at 1000K
\(CO_{2}(g)\rightleftharpoons C(s)+O_{2}(G) K_{eq}\)= \(1\times 10^{-32} \)at 1000K
The formation of Fe(s) and\( O_2\)(g) from FeO(s) is not thermodynamically favorable at room temperature. In an effort to make the process favorable, C(s) is added to the FeO(s) at elevated temperatures. Based on the information above, which of the following gives the value of K eq and the sign of \(\Delta G^{\circ}\) for the reaction represented by the equation below at 1000 K?
$2FeO(s)+ C_{s} \rightleftharpoons 2Fe(s)+CO_{2}$
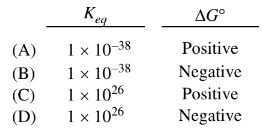
▶️Answer/Explanation
Ans: D
Given:
1. \(K_{eq}\) for \(2FeO(s) \rightleftharpoons 2Fe(s) + O_{2}\) is \(1 \times 10^{-6}\) at 1000 K.
2. \(K_{eq}\) for \(CO_{2}(g) \rightleftharpoons C(s) + O_{2}(g)\) is \(1 \times 10^{-32}\) at 1000 K.
To find the equilibrium constant \(K_{eq}\) for the overall reaction:
\[ 2FeO(s) + C_{s} \rightleftharpoons 2Fe(s) + CO_{2} \]
We need to consider the equilibrium constant for the individual reactions and apply them to determine the equilibrium constant for the overall reaction.
Given the equilibrium constants for the individual reactions, we know:
The reverse reactions are favored at 1000 K for both reactions because their equilibrium constants are very small.
This suggests that the forward reactions are thermodynamically unfavorable.
For the overall reaction, it involves the combination of two unfavorable forward reactions. Thus, the overall equilibrium constant will be extremely small, as it is the product of the equilibrium constants of the
individual reactions.
\[K_{eq}=K_{1} \times K_{2} = (1 times 10^{-6}) \times (1 \times 10^{32}) =1 \times 10^{26} \]
Given that the overall reaction proceeds in the thermodynamically unfavorable direction, the sign of \(AG^{\circ}\) for the overall reaction will be negative.
Therefore, the correct answer is:
(D) \(1 \times 10^{26}\) & Negative
Questions
\(H_{2}(g)+I_{2}(g)\rightleftharpoons 2HI(g)\)
Hydrogen gas reacts with iodine gas at constant temperature in a sealed rigid container. The gases are allowed to reach equilibrium according to the equation above. Which of the following best describes what will happen to the reaction immediately after additional iodine gas is added to the system?
(A) The rates of both the forward and reverse reactions decrease.
(B) The rates of both the forward and reverse reactions do not change.
(C) The rate of the forward reaction becomes greater than the rate of the reverse reaction.
(D) The rate of the forward reaction becomes less than the rate of the reverse reaction.
▶️Answer/Explanation
Ans: C
When additional iodine gas (\(I_2\)) is added to the system, according to Le Chatelier’s Principle, the system will shift to counteract the change. Since iodine is a reactant in the forward reaction, increasing its concentration will shift the equilibrium position to the right to consume some of the added iodine.
To restore equilibrium, the system will favor the forward reaction to consume the excess iodine gas. Therefore, the rate of the forward reaction will momentarily increase to consume the added \(I_2\), while the rate of the reverse reaction will remain unaffected initially.
Hence, the best description of what will happen to the reaction immediately after additional iodine gas is added to the system is:
(C) The rate of the forward reaction becomes greater than the rate of the reverse reaction.
Questions
\(N_{2}O_{4(g)}\rightleftharpoons 2NO_{2(g)}\) \(K_{p}=3.0 at 70^{\circ C}\)
colorless brown
A mixture of \(NO_{2(g)}\) and \(N_{2}O_{4(g)}\) is placed in a glass tube and allowed to reach equilibrium at \(70^{\circ C}\), as represented above. Which of the following statements best helps to explain why the contents of the tube containing the equilibrium mixture turned a lighter color when the tube was placed into an ice bath?
(A) The forward reaction is exothermic.
(B) The forward reaction is endothermic.
(C) The ice bath lowered the activation energy.
(D) The ice bath raised the activation energy.
▶️Answer/Explanation
Ans: B
The color change in the equilibrium mixture when placed in an ice bath suggests a shift in the equilibrium position. Let’s analyze the options:
A. The forward reaction is exothermic.
- If the forward reaction were exothermic, removing heat by placing the tube in an ice bath would shift the equilibrium towards the reactants to counteract the decrease in temperature. This would lead to an increase in the concentration of N₂O₄, which is brown. However, the lighter color observed suggests an increase in the concentration of NO₂, the colorless gas. Therefore, option (A) is not the best explanation.
B. The forward reaction is endothermic.
- This option suggests that the forward reaction requires the absorption of heat. Placing the tube in an ice bath would favor the endothermic forward reaction to counteract the decrease in temperature, resulting in an increase in the concentration of the colorless NO₂. This is consistent with the observed lighter color. Hence, option (B) is the best explanation.
C. The ice bath lowered the activation energy.
- This statement is not directly related to the observed color change in the equilibrium mixture. Lowering the activation energy could affect the rate of the reaction, but it doesn’t explain why the color changed in the equilibrium mixture.
D. The ice bath raised the activation energy.
- Similarly to option (C), this statement is not directly related to the observed color change. Raising the activation energy could potentially slow down the reaction, but it doesn’t explain the shift in equilibrium leading to the color change.
Therefore, the best explanation for the color change is option (B) – the forward reaction is endothermic.
Question
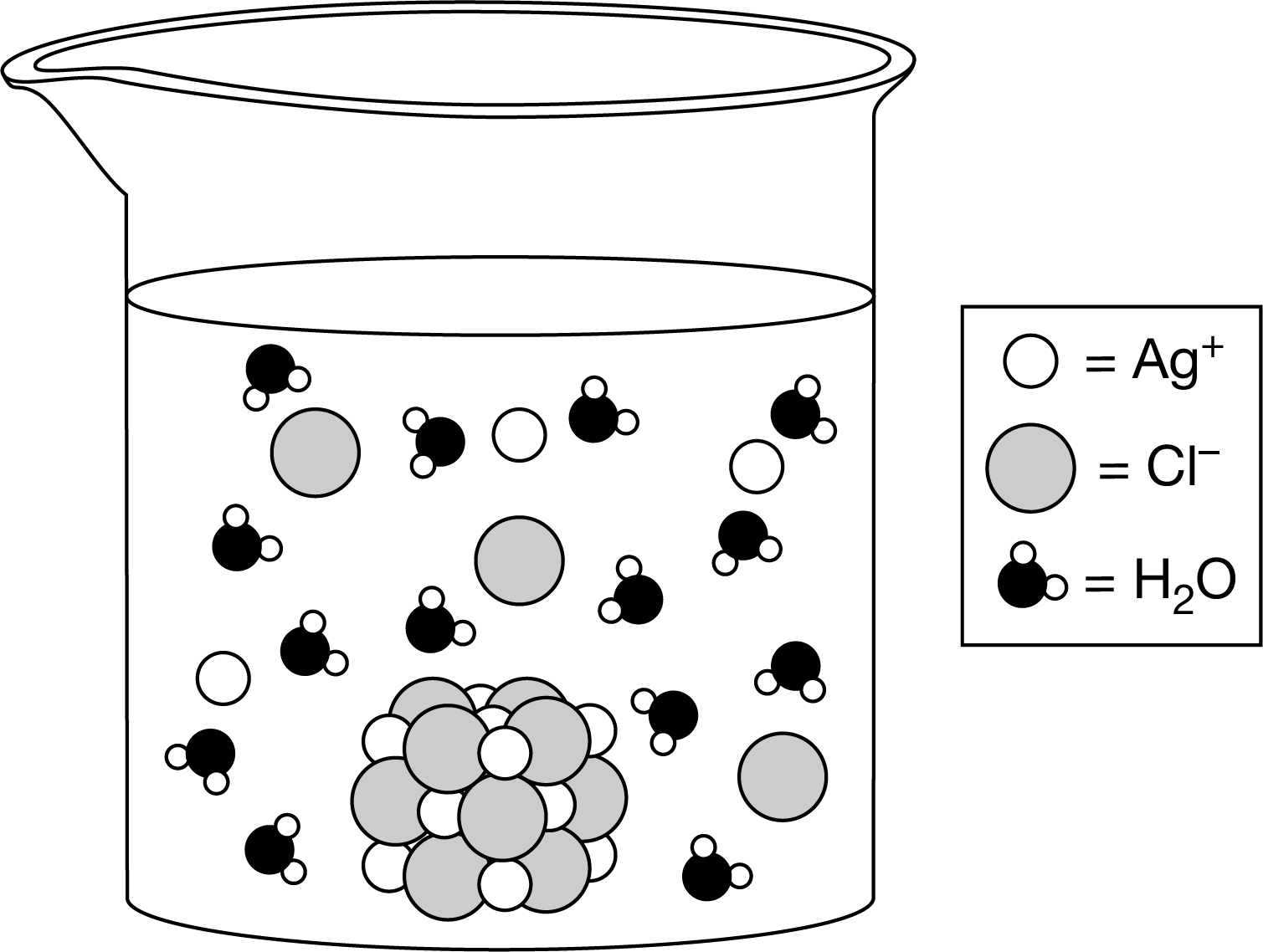
\(AgCl(s)⇄Ag^+(aq)+Cl^−(aq)\)
When \(AgCl(s)\) is placed in water, it dissolves according to the chemical equation above. The particle diagram above was proposed to represent an aqueous solution in which \(AgCl(s)\)is in equilibrium with its ions. Which of the following best explains whether or not the diagram provides a good representation of this dynamic equilibrium at the microscopic level?
A It is a good representation because it shows that the dipoles of the \(H_2O\) molecules are oriented around the \(Ag^+\) and \(Cl^−\) ions in solution.
B It is a good representation because it shows that the concentrations of \(Ag^+\) ions and \(Cl^−\) ions are equal at equilibrium.
C It is not a good representation because it does not show that the concentration of \(AgCl(s)\) is constant at equilibrium.
D It is not a good representation because it does not illustrate the dynamic equilibrium in which the rates of the forward and reverse reactions are equal.
▶️Answer/Explanation
Ans:D
Since at equilibrium the rates of the forward and reverse reactions are the same, one single representation cannot capture the dynamic nature of this process. Multiple representations of the reaction before and after equilibrium would be needed to show that the concentrations of the ions have remained constant over time.
Question
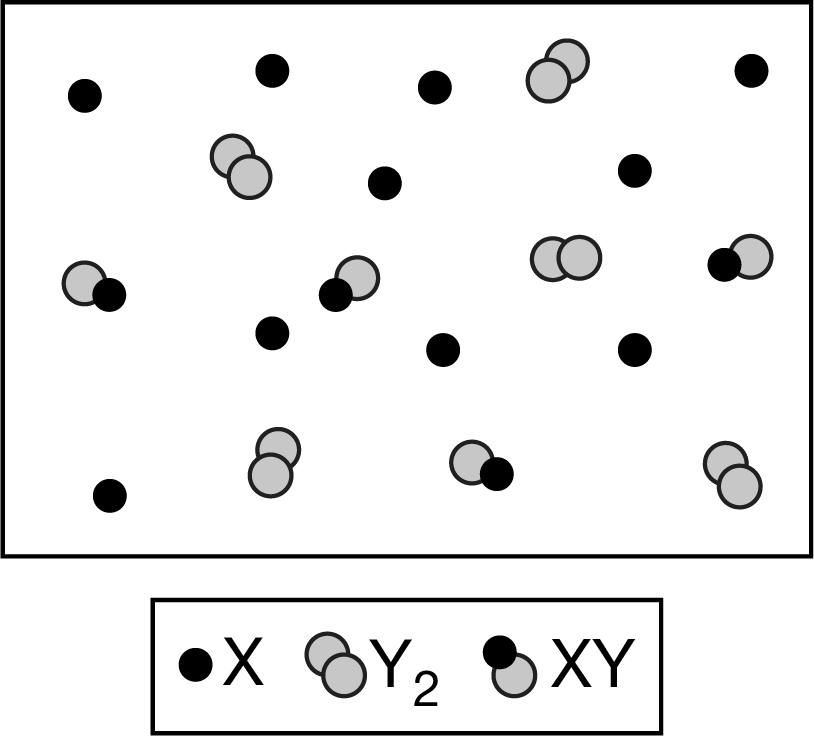
The particle diagram above shows the system represented by the equation \(2X(g)+Y_2(g)\)⇄\(2XY(g)\) . Which of the following explains whether the particle diagram indicates that the system is at equilibrium?
A The particle diagram does not indicate that the system is at equilibrium because it shows the system only at one point in time.
B The particle diagram does not indicate that the system is at equilibrium because the ratio \(\frac{[XY]_{eq}}{[X]_{eq}}\) is not equal to 1.
C The particle diagram indicates that the system is at equilibrium because the value of K is small.
D The particle diagram indicates that the system is at equilibrium because \([XY]_{eq}=2×[Y_2]_{eq}\).
▶️Answer/Explanation
Ans:A
Equilibrium is a dynamic process in which the rates of the forward reaction and the reverse reaction are equal and the concentrations of reactants and products remain constant over time. A single representation cannot capture this dynamic process.
Question
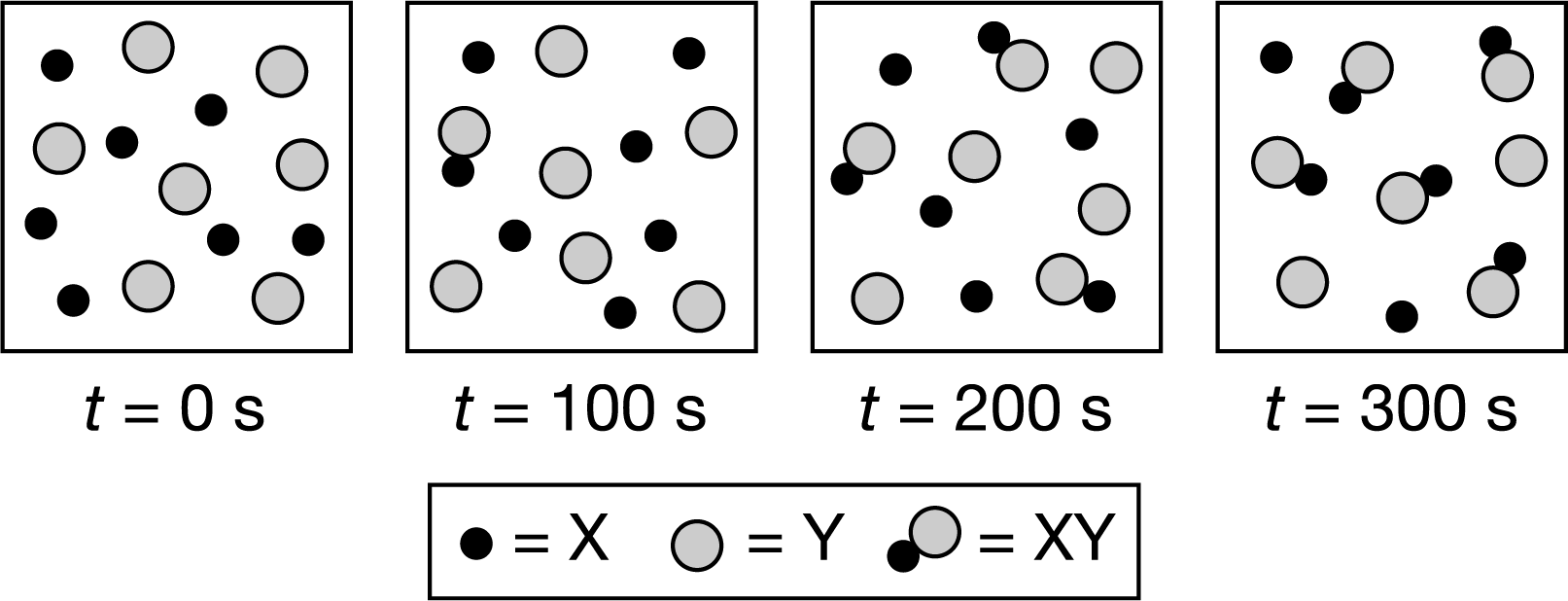
\(X(g)+Y(g)\)⇄\(XY(g)\)
The particle diagrams above show the changes that occurred after an equimolecular mixture of X(g) and Y(g) was placed inside a rigid container at constant temperature. Which of the following statements is be best supported by the particle diagrams?
A The rate of the reverse reaction is zero because the concentration of XY keeps increasing.
B The reverse reaction has a higher rate than the forward reaction between 200s and 300s because [XY]>>[X].
C The forward reaction has a faster rate than the reverse reaction between 0s and 300s because more products were being formed.
D The rates of the forward and reverse reactions were the same after 100s because the rate of formation of XY was constant.
▶️Answer/Explanation
Ans:C
Based on the particle diagrams, the amount of XY kept increasing between 0s and 300s, indicating that the forward reaction occurred at a faster rate than the reverse reaction.
Question
\(X_{2}+Y_{2}(g)\rightleftharpoons 2XY(g) k_{c}=3.0\)
A mixture of\( X_{2}\)(g), \(Y_2\)(g), and XY(g) is placed in a previously evacuated, rigid container and allowed to reach equilibrium at a constant temperature, as shown above. Which of the following sets of initial concentrations would lead to the formation of more product as the system moves toward equilibrium?

▶️Answer/Explanation
Ans:A
Question

Which of the following happens to H atoms in the forward reaction?
(A) H atoms are oxidized only.
(B) H atoms are reduced only.
(C) H atoms are both oxidized and reduced.
(D) H atoms are neither oxidized nor reduced.
▶️Answer/Explanation
Ans:C