Questions
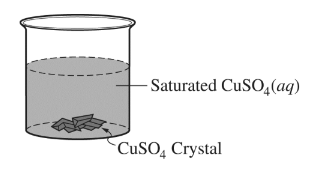
The saturated\( CuSO_4\)(aq) shown above is left uncovered on a lab bench at a constant temperature. As the solution evaporates, 1.0 mL samples of the solution are removed every three days and the \([SO_{4} ^{2-}]\) in the samples is measured. It is observed that the \([SO_{4}^{2-}]\) in the solution did not change over time. Which of the following best helps to explain the observation?
(A) As the solution evaporates, \(Cu^{2+}\)(aq) and \(SO_{4} ^{2-}\)(aq) leave the beaker along with the water molecules.
(B) As the solution evaporates, the dissolution of \(CuSO_{4}\)(s) in the beaker decreases.
(C) The evaporation of water is endothermic, so more \(CuSO_{4}\)(s) dissolves exothermically in the solution, which increases the\( [SO_{4}^{2-}]\).
(D) As water evaporates, more\( CuSO_{4}\)(s) precipitates out of the solution in the beaker.
▶️Answer/Explanation
Ans: B
The observation that the concentration of \( SO_{4}^{2-} \) in the solution remains constant despite the evaporation of water suggests that the dissolution of \( CuSO_{4}(s) \) in the beaker is a key factor. Let’s analyze each option:
(A) This statement suggests that both \( Cu^{2+} \) and \( SO_{4}^{2-} \) ions leave the solution along with water molecules as it evaporates. However, this contradicts the premise that the concentration of \( SO_{4}^{2-} \) remains constant.
(B) This option aligns with the observation. If the dissolution of \( CuSO_{4}(s) \) in the beaker decreases as the solution evaporates, the concentration of \( SO_{4}^{2-} \) would remain constant because there’s no additional source of sulfate ions in the system.
(C) This option suggests that the evaporation of water causes more \( CuSO_{4}(s) \) to dissolve in the solution, which increases the \( SO_{4}^{2-} \) concentration. However, this is unlikely as the dissolution of \( CuSO_{4}(s) \) generally decreases with decreasing solution volume due to saturation.
(D) This option proposes that as water evaporates, more \( CuSO_{4}(s) \) precipitates out of the solution. However, if this were the case, the concentration of \( SO_{4}^{2-} \) would decrease over time, which contradicts the observation.
Therefore, option (B) is the best explanation for the observation, as it suggests that the decrease in the dissolution of \( CuSO_{4}(s) \) in the beaker is responsible for the constant concentration of \( SO_{4}^{2-} \) in the solution.
Questions
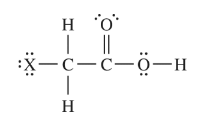
The structure of haloacetic acids, \(XCH_{2}COOH\) (where X is either F, Cl, Br, or I ), is shown above. The dissociation constants and
molar masses of four haloacetic acids are listed in the table below.
A student titrates 10.0 mL samples of 1.0 M solutions of each of the haloacetic acids with a standard solution of NaOH. Which of the
following statements correctly predicts the volume of NaOH(aq) needed to reach the equivalence point?
(A) Fluoroacetic acid will need the smallest volume of NaOH(aq) to reach the equivalence point.
(B) Iodoacetic acid will need the smallest volume of NaOH(aq) to reach the equivalence point.
(C) All of the acids will need the same volume of NaOH(aq) to reach the equivalence point.
(D) All of the haloacetic acids are weak; therefore none will reach an equivalence point.
▶️Answer/Explanation
Ans: A
To predict the volume of NaOH(aq) needed to reach the equivalence point in each titration, we need to consider the dissociation constants (\(Ka\)) of the haloacetic acids.
The dissociation constant (\(Ka\)) represents the extent to which an acid dissociates in solution. The stronger the acid (higher \(Ka\)), the more it dissociates, requiring less volume of NaOH to neutralize.
Given that the haloacetic acids are weak acids, they will undergo partial dissociation in solution. However, the \(Ka\) values can still give us an indication of their relative strengths.
Looking at the options:
(A) Fluoroacetic acid will need the smallest volume of NaOH(aq) to reach the equivalence point.
(B) Iodoacetic acid will need the smallest volume of NaOH(aq) to reach the equivalence point.
(C) All of the acids will need the same volume of NaOH(aq) to reach the equivalence point.
(D) All of the haloacetic acids are weak; therefore none will reach an equivalence point.
We can rule out option (C) because the acids have different dissociation constants, so they won’t require the same volume of NaOH.
Option (D) is incorrect because even weak acids can reach an equivalence point in a titration. The equivalence point is reached when the moles of acid are equal to the moles of base added, irrespective of the
strength of the acid.
To determine between options (A) and (B), we need to compare the \(Ka\) values of fluoroacetic acid and iodoacetic acid. The acid with the higher \(Ka\) value will be stronger and will require less volume of
NaOH to reach the equivalence point.
If we assume that the haloacetic acids follow the trend of increasing acidity with increasing atomic number of the halogen (i.e., fluorine is the most electronegative and iodine is the least electronegative), then
the trend in \(K a\) values might be as follows:
$V[K a(\text{Fluoroacetic acid}) > Ka(\text{Chloroacetic acid}) > Ka(\text{Bromoacetic acid}) > Ka(\text{Iodoacetic acid})]$
So, option (A) is correct: Fluoroacetic acid will need the smallest volume of NaOH(aq) to reach the equivalence point.
Question
A group of students was asked to recover Cu(s) from a blue-green aqueous solution containing an unknown concentration of \(Cu^{2+}\)(aq). The students took a 100.0 mL sample of the solution and added an excess of 1.0 \(M Na_3PO_4\)(aq), causing the\( Cu^{2+}\)(aq) to precipitate as Cu3(PO4)2(s), as shown in step 1 below.
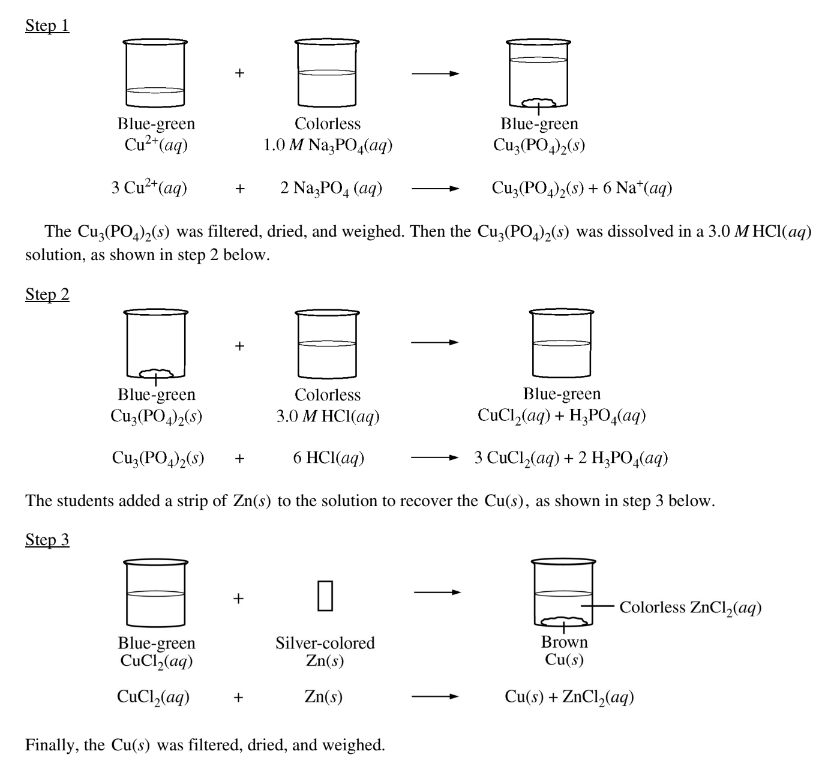
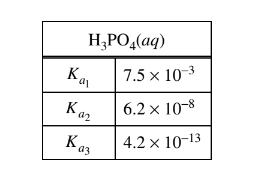
The values of the dissociation constants for \(H_{3}PO_{4}\) are given in the table above. Given thatmthe solution in the beaker at the end of step 2 had a pH of approximately 1, which of the following chemical species had the lowest concentration among the products of step 2 ?
(A) \( H^{+}\)(aq)
(B) \(H_{2}PO_{4 }^{-}(aq) \)
(C) \(HPO_{4}^{2−}\)(aq)
(D) \(PO_{4}^{3−}\)(aq)
▶️Answer/Explanation
Ans:D
To determine which chemical species had the lowest concentration among the products of step 2, we can analyze the dissociation of \(\mathrm{H}_3\mathrm{PO}_4\) and consider their equilibrium concentrations at a pH of approximately 1.
\(\mathrm{H}_3\mathrm{PO}_4\) dissociates in a stepwise manner as follows:
1. \(\mathrm{H}_3\mathrm{PO}_4 \rightleftharpoons \mathrm{H}^+ + \mathrm{H}_2\mathrm{PO}_4^-\) (First dissociation)
2. \(\mathrm{H}_2\mathrm{PO}_4^- \rightleftharpoons \mathrm{H}^+ + \mathrm{H}\mathrm{PO}_4^{2-}\) (Second dissociation)
3. \(\mathrm{HPO}_4^{2-} \rightleftharpoons \mathrm{H}^+ + \mathrm{PO}_4^{3-}\) (Third dissociation)
At a pH of approximately 1, we can expect that the first dissociation (\(\mathrm{H}_3\mathrm{PO}_4 \rightleftharpoons \mathrm{H}^+ + \mathrm{H}_2\mathrm{PO}_4^-\)) will be essentially complete, and the concentrations of \(\mathrm{H}_2\mathrm{PO}_4^-\), \(\mathrm{HPO}_4^{2-}\), and \(\mathrm{PO}_4^{3-}\) will be relatively low.
Comparing the equilibrium concentrations:
The concentration of \(\mathrm{H}^+\) will be high since the solution has a pH of approximately 1.
The concentration of \(\mathrm{H}_2\mathrm{PO}_4^-\) will be higher than that of \(\mathrm{HPO}_4^{2-}\) and \(\mathrm{PO}_4^{3-}\) because it is the first product of the dissociation process.
The concentration of \(\mathrm{HPO}_4^{2-}\) will be higher than that of \(\mathrm{PO}_4^{3-}\) because it is the second product of the dissociation process.
Therefore, among the given options, the chemical species with the lowest concentration among the products of step 2 is \(\mathrm{PO}_4^{3-}\)(aq) (option D).
Question
For which of the following salts would the relationship between molar solubility, \(s\), in mol/L, and the value of \(K_{sp}\) be represented by the equation \(K_{sp}=4s^3\) ?
A \(PbCO_3\)
B \(Mg_3(PO_4)_2\)
C \(Ag_2SO_4\)
D \(MnS\)
▶️Answer/Explanation
Ans:C
\(Ag_2SO_4\) dissolves to form \(2Ag^+\)(aq) ions and 1 \(SO_4^{2−}\)(aq) ion. Letting s represent the molar solubility of \(Ag_2SO_4\), the Ksp expression for \(Ag_2SO_4\) is \(K_{sp}=[Ag^+]^{2}[SO_4^{2−}]=(2s)^2(s)=4s^3\).
Question
\(Hg_2I_2\)(s)⇄\(Hg_2^{2+}(aq)+2 I^−(aq)\) \(K_{sp}=[Hg_2^{2+}][I^−]^2\)
A saturated solution of \(Hg_2I_2\) is at equilibrium at 25°C as represented by the equation above. If \([I^−]=4.6×10^{−10}\) M at equilibrium, which of the following gives the correct molar solubility, S, and \(K_{sp}\) for \(Hg_2I_2\) ?
A \(S=4.6×10^{−10}\) M; \(K_{sp}=(2.3×10^{−10})(4.6×10^{−10})^2\)
B \(S=4.6×10^{−10}\)M; \(K_{sp}=(4.6×10^{−10})(9.2×10^{−10})^2\)
C \(S=2.3×10^{−10}\)M; \(K_{sp}=(2.3×10^{−10})(4.6×10−10)^2\)
D \(S=2.3×10^{−10}\)M; \(K_{sp}=(4.6×10^{−10})(9.2×10^{−10})^2\)
▶️Answer/Explanation
Ans:C
Based on the stoichiometry of the reaction, \(S=[Hg_2^{2+}]=\frac{[I^−]}{2}\) . Therefore, \(S=2.3×10^{−10}\)M; \(K_{sp}=(2.3×10^{−10})(4.6×10−10)^2\).
Question
\(AgI\)(s)⇄\(Ag^+(aq)+I^−(aq)\) \(K_{sp}=8.3×10^{−17}\) at298K
The dissolution of \(AgI\) is represented above. Which of the following shows the mathematical relationship between the molar solubility, \(S\), and the \(K_{sp}\) of \(AgI\) at 298K ?
A \(S=8.3×10^{−17}mol/L\)
B \(S=(\frac{8.3×10^{−17}}{2})mol/L\)
C \(S=\sqrt{8.3\times10^{-17}}mol/L\)
D \(S=2\sqrt{8.3\times10^{-17}}mol/L\)
▶️Answer/Explanation
Ans:C
Since \(AgI\) dissociates to form one mole of \(Ag^+\)(aq) and one mole of \(I^−\)(aq), \(K_{sp}=[Ag^+][I^−]=8.3×10^{−17}\) and the molar solubility of \(AgI =[Ag^+] =[I^−] =\sqrt{8.3\times10^{-17}}mol/L\).
Question
Which of the following best helps to explain why the value of \(\Delta H^{\circ}\) for the dissolving of \(CaF_2\) in water is positive?
(A)\( CaF_{2}(s)\) is insoluble in water.
(B) \(CaF_{2}(s)\) dissolves in water to form \(CaF_{2}\)(aq) particles.
(C) \(Ca^{2+}\) ions have very strong ion-ion interactions with F – ions in the crystal lattice.
(D) \(Ca^{2+}\) ions have very strong ion-dipole interactions with water molecules in the solution.
▶️Answer/Explanation
Ans:C
Question
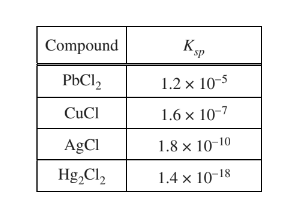
Based on the Ksp values in the table above, a saturated solution of which of the following compounds has the highest \({cl ^-}\) ?
(A) \(PbCl_{2}\)
(B) CuCl
(C) AgCl
(D) \(Hg_{2}Cl_{2}\)
▶️Answer/Explanation
Ans:B