Questions

A mixture of\( H_{2}\)(g) and \(O_{2}\)(g) is placed in a container as represented above. The \(H_2\)(g) and \(O _{2}\)(g) react to form \(H_{2}\)O(g). Which of the
following best represents the container after the reaction has gone to completion?

▶️Answer/Explanation
Ans: D
Answer of above question is (D) as it is showing exact molecules of water containing $H_2O$ in container and also showing hydrogen bonding .
Question
26. Which of the following best helps to explain why \( CCl_4\) is a liquid whereas \( CI_4\) is a solid when both
are at 25°C?
(A) The dipole moment of the\( CCl_4\) molecule is larger than that of the\( CI_4\) molecule because Cl is more electronegative than I.
(B) The dipole moment of the \( CI_4\) molecule is larger than that of the \( CCl_4\) molecule because there is stronger repulsion between electrons in the C−I bonds compared to the repulsion between electrons in the C−Cl
bonds.
(C) The London dispersion forces are stronger in \( CCl_4\) than in\( CI_4\) because Cl is more electronegative than I.
(D) The London dispersion forces are stronger in \( CI_4\) than in \( CCl_4\) because \( CI_4\) has a more polarizable electron cloud than \( CCl_4\) .
▶️Answer/Explanation
Ans:D
The difference in physical states (liquid vs. solid) between \( CCl_4 \) (carbon tetrachloride) and \( CI_4 \) (carbon tetraiodide) at 25°C can be attributed to the intermolecular forces present in each compound.
In \( CCl_4 \), the carbon atom is bonded to four chlorine atoms, creating a symmetric tetrahedral structure. Chlorine is more electronegative than carbon, resulting in polar covalent C-Cl bonds. While the molecule as a whole is nonpolar due to its symmetric geometry, there are still London dispersion forces present between \( CCl_4 \) molecules due to temporary fluctuations in electron density.
In \( CI_4 \), the carbon atom is bonded to four iodine atoms. Iodine is also more electronegative than carbon, resulting in polar covalent C-I bonds. However, due to the larger size of iodine atoms compared to chlorine atoms, the molecule is more polarizable, meaning that the electron cloud is more easily distorted. This leads to stronger London dispersion forces between \( CI_4 \) molecules compared to \( CCl_4 \) molecules.
Given this information, we can eliminate options (A) and (B) because they incorrectly discuss dipole moments rather than considering London dispersion forces, which are the primary intermolecular forces at play in nonpolar molecules like \( CCl_4 \) and \( CI_4 \).
Option (C) suggests that the London dispersion forces are stronger in \( CCl_4 \) than in \( CI_4 \) because chlorine is more electronegative than iodine. However, this statement is incorrect because the strength of London dispersion forces primarily depends on the polarizability of the electron cloud, not the electronegativity of the atoms. Therefore, option (C) is incorrect.
Option (D) correctly explains that the London dispersion forces are stronger in \( CI_4 \) than in \( CCl_4 \) because \( CI_4 \) has a more polarizable electron cloud due to the larger size of iodine atoms compared to chlorine atoms. This greater polarizability results in stronger London dispersion forces in \( CI_4 \), which contributes to its solid state at 25°C compared to the liquid state of \( CCl_4 \). Therefore, option (D) is the best explanation.
Questions

A mixture of \(Co_({g})\) and \(O_{2(g)}\) is placed in a container, as shown above. A reaction occurs, forming \(Co_{2(g)}\). Which of the following best represents the contents of the box after the reaction has proceeded as completely as possible?

▶️Answer/Explanation
Ans: B
Option (b) accurately represents the contents of the container after the reaction \( \text{Co(g)} + \frac{1}{2} \text{O}_2(\text{g}) \rightarrow \text{Co}_2(\text{g}) \) has proceeded as completely as possible.
In the initial mixture, there are multiple \( \text{Co(g)} \) molecules (gray circles) and multiple \( \text{O}_2(\text{g}) \) molecules (white circles with a black dot). The reaction consumes \( \text{Co(g)} \) and \( \text{O}_2(\text{g}) \) in a 2:1 ratio to form \( \text{Co}_2(\text{g}) \) (two connected gray circles).
Option (b) shows a mixture containing:
Several \( \text{Co}_2(\text{g}) \) molecules, indicating that the reaction has occurred and consumed some of the \( \text{Co(g)} \) and \( \text{O}_2(\text{g}) \) reactants.
Some remaining unreacted \( \text{Co(g)} \) molecules (individual gray circles).
Some remaining unreacted \( \text{O}_2(\text{g}) \) molecules (white circles with a black dot).
This correctly represents the scenario where the reaction has proceeded as far as possible, consuming the available reactants in the 2:1 ratio, but leaving behind any excess unreacted \( \text{Co(g)} \) and \( \text{O}_2(\text{g}) \) molecules that could not form complete \( \text{Co}_2(\text{g}) \) units due to the limiting reactant being depleted.
The other options either show no reaction occurring at all ((a) and (c)), or indicate that the reaction went to complete consumption of all reactants ((d)), which contradicts the information that some unreacted molecules should remain after the reaction proceeds as completely as possible.
Question
A student combines a solution of \(NaCl\)(aq) with a solution of \(AgNO_3\)(aq) , and a precipitate forms. Which of the following is evidence that ionic bonds formed during the precipitation?
A The resulting solution is colorless.
B The resulting solution conducts electricity.
C The precipitate has a high melting point.
D The temperature of the solution did not change significantly during the precipitation.
▶️Answer/Explanation
Ans:C
A characteristic of compounds containing ionic bonding is a high melting point.
Question
A beaker was half filled with freshly distilled \(H_2O\) and placed on a hot plate. As the temperature of the water reached 100°C , vigorous bubbling was observed in the beaker. The gaseous contents of the bubbles were analyzed. The presence of which of the following substances would support the claim that the observed phenomenon was a physical change?
A \(H_2(g)\)
B \(O_2(g)\)
C \(CO_2(g)\)
D \(H_2O(g)\)
▶️Answer/Explanation
Ans: D
When water boils, a physical change takes place in which \(H_2O(l)\) changes to \(H_2O(g)\). If the bubbles formed were \(H_2O(g)\), then the phenomenon would be a physical change.
Question
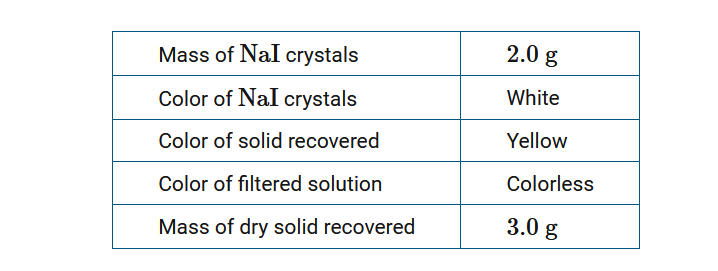
When students added 2.0g of NaI crystals to 100.mL of \(Pb(NO_3)_2(aq)\) , a yellow precipitate formed. After the solution was filtered, the yellow solid was dried and weighed. Data from the experiment are shown in the table above. Which of the following claims is best supported by the observations?
A A physical change occurred when a new yellow compound was formed.
B A physical change occurred when the color of the NaI solid added changed to yellow when mixed with water.
C A chemical change occurred when a yellow, insoluble compound with a larger mass than the original NaI formed.
D A chemical change occurred when covalent bonds between the yellow solid and water were broken during drying.
▶️Answer/Explanation
Ans:C
A chemical change occurred because of the formation of a new yellow, insoluble compound with a larger mass than that of NaI.
Refer to the following.
\( M^{+}\) is an unknown metal cation with a +1 charge. A student dissolves the chloride of the unknown metal, MCI, in enough water to make 100.0 mL of solution. The student then mixes the solution with excess \(AgN0_3\) solution, causing AgCl to precipitate. The student collects the precipitate by filtration, dries it, and records the data shown below. (The molar mass of AgCl is 143 g/mol.)
Mass of unknown chloride, MCI 0.74 g
Mass of filter paper 0.80 g
Mass of filter paper plus AgCl precipitate 2.23 g
Question
Which of the following diagrams best represents the \(AgNO_{3}\) solution before the reaction occurs? Note: water molecules are represented by the symbol
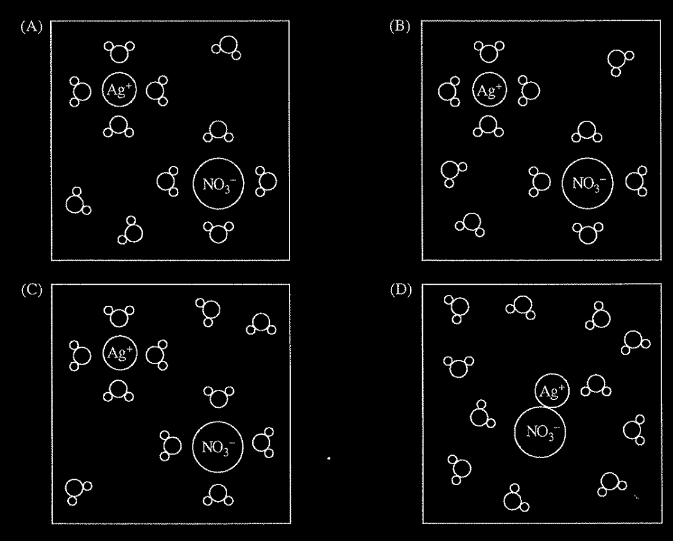
▶️Answer/Explanation
Ans:A
Refer to the following.
\( M^{+}\) is an unknown metal cation with a +1 charge. A student dissolves the chloride of the unknown metal, MCI, in enough water to make 100.0 mL of solution. The student then mixes the solution with excess \(AgN0_3\) solution, causing AgCl to precipitate. The student collects the precipitate by filtration, dries it, and records the data shown below. (The molar mass of AgCl is 143 g/mol.)
Mass of unknown chloride, MCI 0.74 g
Mass of filter paper 0.80 g
Mass of filter paper plus AgCl precipitate 2.23 g
Question
Which of the following diagrams best represents the \(AgNO_{3}\) solution before the reaction occurs? Note: water molecules are represented by the symbol

▶️Answer/Explanation
Ans:A