Question
\(CH_3CH_2COOH(aq) + H_2O(l) \rightleftharpoons CH_3CH_2COO^ ̄(aq) + H2O^{+}(aq) \)
Propanoic acid, \( CH_3CH_2COOH\), is a carboxylic acid that reacts with water according to the equation above. At 25°C the pH of a 50.0 mL sample of 0.20 M \(CH_3CH_2COOH \) is 2.79.
(a) Identify a Brønsted-Lowry conjugate acid-base pair in the reaction. Clearly label which is the acid and which is the base.
(b) Determine the value of K for propanoic acid at 25°C.
(c) For each of the following statements, determine whether the statement is true or false. In each case, explain the reasoning that supports your answer. (i) The pH of a solution prepared by mixing the 50.0 mL sample of 0.20 M \(CH_3CH_2COOH\) with a 50.0 mL sample of 0.20 M NaOH is 7.00. (ii) If the pH of a hydrochloric acid solution is the same as the pH of a propanoic acid solution, then the molar concentration of the hydrochloric acid solution must be less than the molar concentration of the propanoic acid solution. A student is given the task of determining the concentration of a propanoic acid solution of unknown concentration. A 0.173 M NaOH solution is available to use as the titrant. The student uses a 25.00 mL volumetric pipet to deliver the propanoic acid solution to a clean, dry flask. After adding an appropriate indicator to the flask, the student titrates the solution with the 0.173 M NaOH, reaching the end point after 20.52 mL of the base solution has been added.
(d) Calculate the molarity of the propanoic acid solution.
(e) The student is asked to redesign the experiment to determine the concentration of a butanoic acid solution instead of a propanoic acid solution. For butanoic acid the value of pK is 4.83. The student claims that a different indicator will be required to determine the equivalence point of the titration accurately. Based on your response to part (b), do you agree with the student’s claim? Justify your answer.
▶️Answer/Explanation
(a) \(CH_3CH_2COOH\) and \(CH _3CH_2COO ^-\)
acid base
OR
\(H_2O^+\) and \(H_2O\)
acid base
(b) \([H_3O^+]=10^{-pH}=10^{-2.79}=1.6\times 10^{-3}\) M
\([CH _3CH_2COO^ ̄]=[H_3O^+]\)
AND
\([CH _3CH_2COOH]=20M-[H_3O^+]\) , OR \([CH _3CH_2COOH]\approx 0.20 M\)
\(K_a=\frac{[CH _3CH_2COO^ ̄][H_3O^+]}{[CH _3CH_2COOH]}=\frac{(1.6\times 10^{-3}M)^2}{0.20 M}\)
c.(i) False. The conjugate base of a weak acid undergoes hydrolysis (see equation below) at equivalence to form a solution with a pH>7.
\(CH _3CH_2COO^ ̄ + H_2O\rightleftharpoons CH_2CH_2COOH + OH ^-\)
c.(ii) True. HCI is a strong acid that ionizes completely. Fewer moles of HCI are needed to produce the same [H3O+] as the propanoic acid solution, which only partially ionizes.
(d) Let x = moles of propanoic acid
then \(x = (0.02052 L NaOH) \times \frac{0.173 mol NaOH}{1 L NaOH}\times \frac{1 mol acid }{1 mol NaOH} = 3.55 \times 10^{-3} mol propanoic acid \Rightarrow \frac{3.55 \times 10^{-3} mol acid}{ 0.02500 L acid} =0.142 M\)
OR
Since \(CH_3CH_2COOH\) is monoprotic and, at the equivalence point, moles \(H^+ = moles OH^-\), then
\(M_AV_A=M_BV_B\)
\(M_A=\frac{M_BV_B}{ V_A}= \frac{(0.173 M NaOH)(20.52 mL NaOH)}{ 25.00 mL acid} =0.142 M\)
(e) Disagree with the student’s claim From part (b) above, \(pK_a\), for propanoic acid is \(log(1.3 × 10^{_5}) = 4.89\). Because 4.83 is so close to 4.89, the pH at the equivalence point in the titration of butanoic acid should be close enough to the pH in the titration of propanoic acid to make the original indicator appropriate for the titration of butanoic acid.
Question
\(CH_3CH_2COOH(aq) + H_2O(l) \rightleftharpoons CH_3CH_2COO^ ̄(aq) + H2O^{+}(aq) \)
Propanoic acid, \( CH_3CH_2COOH\), is a carboxylic acid that reacts with water according to the equation above. At 25°C the pH of a 50.0 mL sample of 0.20 M \(CH_3CH_2COOH \) is 2.79.
(a) Identify a Brønsted-Lowry conjugate acid-base pair in the reaction. Clearly label which is the acid and which is the base.
(b) Determine the value of K for propanoic acid at 25°C.
(c) For each of the following statements, determine whether the statement is true or false. In each case, explain the reasoning that supports your answer. (i) The pH of a solution prepared by mixing the 50.0 mL sample of 0.20 M \(CH_3CH_2COOH\) with a 50.0 mL sample of 0.20 M NaOH is 7.00. (ii) If the pH of a hydrochloric acid solution is the same as the pH of a propanoic acid solution, then the molar concentration of the hydrochloric acid solution must be less than the molar concentration of the propanoic acid solution. A student is given the task of determining the concentration of a propanoic acid solution of unknown concentration. A 0.173 M NaOH solution is available to use as the titrant. The student uses a 25.00 mL volumetric pipet to deliver the propanoic acid solution to a clean, dry flask. After adding an appropriate indicator to the flask, the student titrates the solution with the 0.173 M NaOH, reaching the end point after 20.52 mL of the base solution has been added.
(d) Calculate the molarity of the propanoic acid solution.
(e) The student is asked to redesign the experiment to determine the concentration of a butanoic acid solution instead of a propanoic acid solution. For butanoic acid the value of pK is 4.83. The student claims that a different indicator will be required to determine the equivalence point of the titration accurately. Based on your response to part (b), do you agree with the student’s claim? Justify your answer.
▶️Answer/Explanation
(a) \(CH_3CH_2COOH\) and \(CH _3CH_2COO ^-\)
acid base
OR
\(H_2O^+\) and \(H_2O\)
acid base
(b) \([H_3O^+]=10^{-pH}=10^{-2.79}=1.6\times 10^{-3}\) M
\([CH _3CH_2COO^ ̄]=[H_3O^+]\)
AND
\([CH _3CH_2COOH]=20M-[H_3O^+]\) , OR \([CH _3CH_2COOH]\approx 0.20 M\)
\(K_a=\frac{[CH _3CH_2COO^ ̄][H_3O^+]}{[CH _3CH_2COOH]}=\frac{(1.6\times 10^{-3}M)^2}{0.20 M}\)
c.(i) False. The conjugate base of a weak acid undergoes hydrolysis (see equation below) at equivalence to form a solution with a pH>7.
\(CH _3CH_2COO^ ̄ + H_2O\rightleftharpoons CH_2CH_2COOH + OH ^-\)
c.(ii) True. HCI is a strong acid that ionizes completely. Fewer moles of HCI are needed to produce the same [H3O+] as the propanoic acid solution, which only partially ionizes.
(d) Let x = moles of propanoic acid
then \(x = (0.02052 L NaOH) \times \frac{0.173 mol NaOH}{1 L NaOH}\times \frac{1 mol acid }{1 mol NaOH} = 3.55 \times 10^{-3} mol propanoic acid \Rightarrow \frac{3.55 \times 10^{-3} mol acid}{ 0.02500 L acid} =0.142 M\)
OR
Since \(CH_3CH_2COOH\) is monoprotic and, at the equivalence point, moles \(H^+ = moles OH^-\), then
\(M_AV_A=M_BV_B\)
\(M_A=\frac{M_BV_B}{ V_A}= \frac{(0.173 M NaOH)(20.52 mL NaOH)}{ 25.00 mL acid} =0.142 M\)
(e) Disagree with the student’s claim From part (b) above, \(pK_a\), for propanoic acid is \(log(1.3 × 10^{_5}) = 4.89\). Because 4.83 is so close to 4.89, the pH at the equivalence point in the titration of butanoic acid should be close enough to the pH in the titration of propanoic acid to make the original indicator appropriate for the titration of butanoic acid.
Question
A 25.0-mL sample of a solution of a monoprotic acid is titrated with a 0.115 M NaOH solution.
The titration curve above was obtained. The concentration of the monoprotic acid is about
__________ mol/L.
▶️Answer/Explanation
Answer:
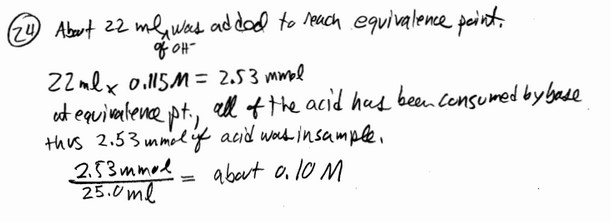
Question
\(NH_3(aq) + H_2O(I) \leftrightarrow NH_4^+(aq) + OH^-(aq)\)
In aqueous solution, ammonia reacts as represented above. In 0.0180 M \(NH_3(aq)\) at \(25^o\)C, the hydroxide ion concentration, [\(OH^ -\)] , is \(5.60 x 10^{-4}\) M. In answering the following, assume that temperature is constant at 25°C and that volumes are additive.
(a) Write the equilibrium-constant expression for the reaction represented above.
(b) Determine the pH of 0.0180 M \(NH_3(aq)\).
(c) Determine the value of the base ionization constant, \(K_b\) , for \(NH_3(aq)\).
(d) Determine the percent ionization of \(NH_3\) in 0.0180 M \(NH_3(aq)\).
(e) In an experiment, a 20.0 mL sample of 0.0180 M \(NH_3(aq)\) was placed in a flask and titrated to the equivalence point and beyond using 0.0120 M HCl(aq).
(i) Determine the volume of 0.0120 M HCl(aq) that was added to reach the equivalence point.
(ii) Determine the pH of the solution in the flask after a total of 15.0 mL of 0.0120 M HCl(aq) was added.
(iii) Determine the pH of the solution in the flask after a total of 40.0 mL of 0.0120 M HCl(aq) was added.
▶️Answer/Explanation
Answer:
(a) \(K = \frac{[NH_4^+][OH^-]}{[NH_3]}\)
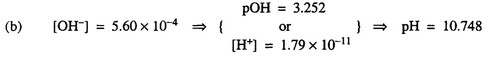
(c) \(K_b =\frac{(5.60 \times 10^{-4})^2}{0.0180-5.60 \times 10^{-4}}= 1.74 \times 10^{-5}\) (or\(1.80 \times 10^{-5}\))
Note : \(1^{st}\) point for \([NH_4^+]=[OH^-]=5.60 \times 10^{-4};2^{nd}\) point for correct answer
(d) % ionization \(=\frac{5.60 \times 10^{-4}}{0.0180} \times 100 % = 6.11 %\) (or 0.311)
(e) \(NH_3 + H^+ \rightarrow NH_4^+\)
(i) mol \(NH_{3} = 0.0180 M \times 0.0200 L = 3.60 \times 10^{-4}~mol =\) mol \(H^+\) needed
vol HCl solution = \(\frac{3.60 \times 10^{-4} mol}{0.0120M}\) = 0.0300 L = 30.0 mL
(ii) mol \(H^+\) added = mol \(0.0120 M \times 0.0150 L = 1.80 \times 10^{-4} mol H^+ added \)
\(= 1.80 \times 10^{-4} mol NH_4^+produced\)
\([NH_4^+]=\frac{1.80 \times 10^{-4}mol}{0.0350 L}=0.00514 M = [NH_3]\)
Note : Point earned for \(1.80 \times 10^{-4}mol\), or \(0.00514 M[NH_3]or[NH_4^+]\),
or statement “halfway to equivalence point”.
\(K_b=1.80 \times 10^{-5} = \frac{[NH_4^+][OH^-]}{[NH_3]}=[ON^-] \Rightarrow pOH = 4.745 \Rightarrow pH = 9.255\)
\((=1.74 \times 10^{-5})(=4.759)(=9.241)\)
(iii) 10.0 mL past equivalence point
\(0.0100 L \times 0.0120 M = 1.20 \times 10^{-4} mol H^+ in 60.0mL\)
\([H^+]=\frac{0.000120mol}{0.0600 L}=0.00200M\)
\(pH = – log(2.00 \times 10^{-3})=2.700\)
