Question
Answer parts (a) through (e) below, which relate to reactions involving silver ion, Ag+.
The reaction between silver ion and solid zinc is represented by the following equation.
2 Ag+(aq) + Zn(s) → Zn2+(aq) + 2 Ag(s)
(a) A 1.50 g sample of Zn is combined with 250. mL of 0.110 M AgNO3 at 250C.
(i) Identify the limiting reactant. Show calculations to support your answer.
(ii) On the basis of the limiting reactant that you identified in part (i), determine the value of [Zn2+] after the reaction is complete. Assume that volume change is negligible.
(b) Determine the value of the standard potential, E0, for a galvanic cell based on the reaction between AgNO3(aq) and solid Zn at 250C.
Another galvanic cell is based on the reaction between Ag+(aq) and Cu(s), represented by the equation below. At 25°C, the standard potential, E0 , for the cell is 0.46 V.
2 Ag+(aq) + Cu(s) →Cu2+(aq) + 2 Ag(s)
(c) Determine the value of the standard free-energy change, ∆G0, for the reaction between Ag+(aq) and Cu(s)
at 250C.
(d) The cell is constructed so that [Cu2+] is 0.045 M and [Ag+] is 0.010 M . Calculate the value of the potential, E0 for the cell.
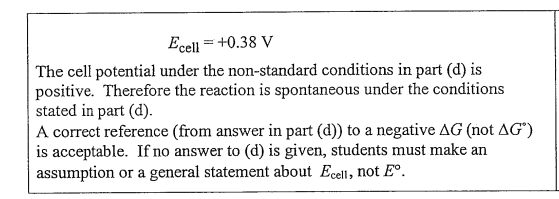
(e) Under the conditions specified in part (d), is the reaction in the cell spontaneous? Justify your answer.
▶️Answer/Explanation
Ans:
(a) (i)
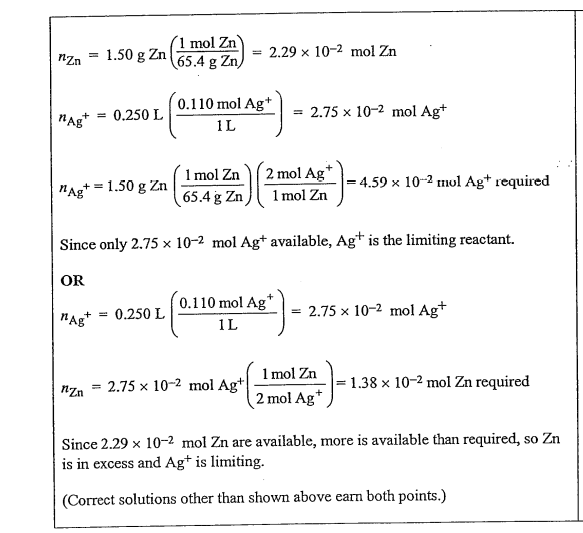
(ii)
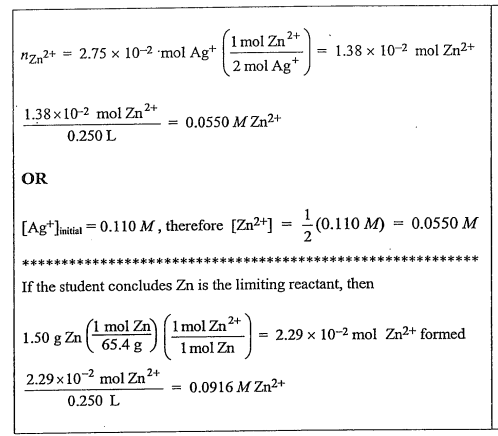
(b)
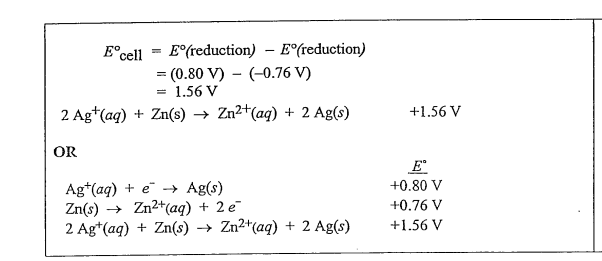
(c)

(d)
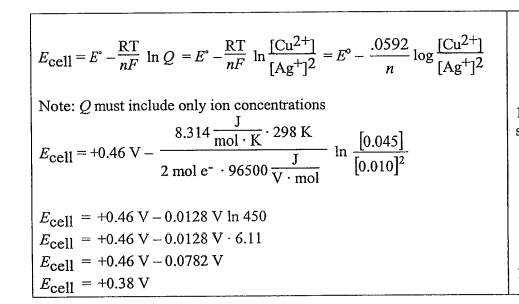
(e)
Question
Answer the following questions relating to the galvanic cell shown in the diagram above.
(a) Write the balanced equation for the overall cell reaction.
(b) Calculate the value of E° for the cell.
(c) Is the value of ΔG° for the overall cell reaction positive, negative, or 0 ? Justify your answer.
(d) Consider the cell as it is operating.
(i) Does \(E_{cell}\) increase, decrease, or remain the same? Explain.
(ii) Does ΔG of the overall cell reaction increase, decrease, or remain the same? Explain.
(iii) What would happen if the NaNO3 solution in the salt bridge was replaced with distilled water? Explain.
(e) After a certain amount of time, the mass of the Ag electrode changes by x grams. Given that the molar mass of Ag is 108 g mol−1 and the molar mass of Co is 59 g mol−1, write the expression for the change in the mass of the Co electrode in terms of x.
▶️Answer/Explanation
Ans:
(a) 2 Ag+(aq) + Co(s) → 2 Ag(s) + Co2+(aq)
(b) \(E_{cell}^{0}=0.80 – (-0.28)=1.08 V\)
(c) The value of ΔG° for the overall reaction must be negative because the cell reaction occurs (is spontaneous) as the cell operates.
OR
Since \(E_{cell}^{0}\) is positive and ΔG° = −nFE°, the value of ΔG° must be negative.
(d) (i) As the cell operates, the concentration of Ag+ decreases and the concentration of Co2+ increases ⇒ the ratio \(Q = \frac{[Co^{2+}]}{[Ag^{+}]^{2}}\) increases ⇒ ln Q increases ⇒ \(E_{cell}=E_{cell}^{0}-\frac{RT}{nF}In Q\) becomes smaller (decreases).
(d)(ii) The value of ΔG for the system increases (becomes less negative) as the cell operates and the system approaches equilibrium (when ΔG = 0).
(d)(iii) The cell would not operate. The voltage of the cell is too small to overcome the electrical resistance of distilled water, which is a poor conductor due to its very low concentration
of ions (about 10−7 M H+(aq) and 10−7 M OH−(aq)) that could “carry” the current from one cell to the other.
(e)
Δ mol Ag = Δ mass \(Ag \times \frac{1 mol Ag}{108 g Ag}=x\times \frac{1}{108}=\frac{x}{108}\)
Δ mol Co = − Δ mol \(Ag \times \frac{1 mol Co}{2 mol Ag}=- \frac{x}{108}\times \frac{1}{2}=-\frac{x}{216}\)
Δ mass Co = Δ mol \(Co\times \frac{59 g Co}{1 mol Co}=- \frac{x}{216}\times 59 = -\frac{59}{216}x\)
