Question

Some binary compounds that form between fluorine and various nonmetals are listed in the table above. A student examines the data in the table and poses the following hypothesis: the number of F atoms that will bond to a nonmetal is always equal to 8 minus the number of valence electrons in the nonmetal atom.
(a) Based on the student’s hypothesis, what should be the formula of the compound that forms between chlorine and fluorine?
(b) In an attempt to verify the hypothesis, the student researches the fluoride compounds of the other halogens and finds the formula \(CIF_{3}\). In the box below, draw a complete Lewis electron-dot diagram for a molecule of\( CIF_{3}\).

(c) Two possible geometric shapes for the\( CIF_{3}\) molecule are trigonal planar and T-shaped. The student does some research and learns that the molecule has a dipole moment. Which of the two shapes is consistent with the fact that the \( CIF_{3}\) molecule has a dipole moment? Justify your answer in terms of bond polarity and molecular structure.
In an attempt to resolve the existence of the \( CIF_{3}\) molecule with the hypothesis stated above, the student researches the compounds that form between halogens and fluorine, and assembles the following list.
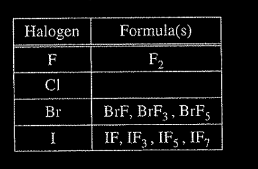
(d) Based on concepts of atomic structure and periodicity, propose a modification to the student’s previous hypothesis to account for the compounds that form between halogens and fluorine.
▶️Answer/Explanation
(a) \(CLF\)
(b) 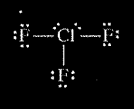
(c) The molecule is T-shaped because a T-shaped structure is asymmetric with dipoles that do not cancel out, but produce a net dipole (i.e., a polar molecule).
OR
because, if the molecule had a trigonal planar structure, the molecule would be symmetric with dipoles that cancel out and produce a net dipole of zero (i.e., a nonpolar molecule), which is not consistent with the observation that the CIF3 molecule does have a dipole moment.
(d) An acceptable hypothesis (descriptive or formulaic) must include the following ideas:
1. Atomic Structure; e.g., odd number of F atoms
2. Periodicity: e.g., as the atomic number of the central halogen atom increases, the number of F atoms increases.
Question.
Propanoic acid, \(C_2H_5COOH\), is an organic acid that is a liquid at room temperature.
(a) An incomplete Lewis diagram for the propanoic acid molecule is provided in the box below. Complete the diagram, showing how the remaining atoms in the molecule are arranged around the carbon atom marked with an asterisk (*). Your structure should minimize formal charge and include any lone pairs of electrons.
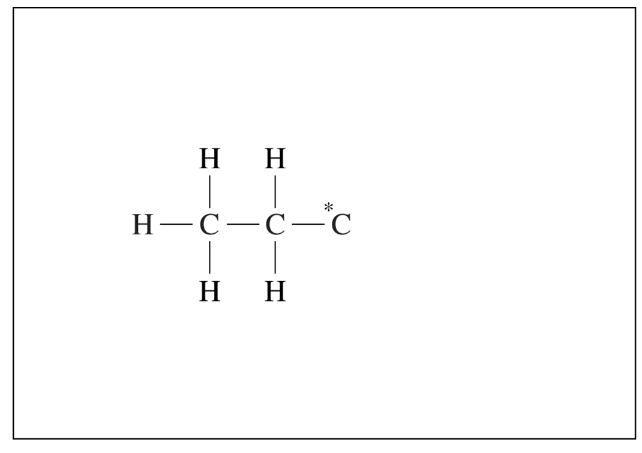
(b) Identify the hybridization of the carbon atom marked with the asterisk.
(c) Propanoic acid has a lower boiling point than butanoic acid, \(C_3H_7COOH\).
(i) Identify all the types of intermolecular forces present among the molecules in propanoic acid.
(ii) Which of the types of intermolecular forces that you identified in part (c)(i) is most responsible for the difference in boiling points of the two acids?
▶️Answer/Explanation
(a) There should be two O atoms attached to the C atom, one with a double bond and one with a single bond. The O atom attached with a single bond should have an H atom attached to it. Each O atom has two lone pairs of electrons. (Figure is required.)
(b) \(sp^2\)
c(i) London dispersion forces, dipole-dipole forces, and hydrogen bonding. (Identifying dipole-dipole forces is not required to earn the point.)
c(ii) London dispersion forces.
Question
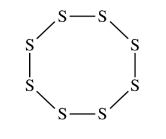
Elemental sulfur can exist as molecules with the formula \(S_8\) . The \(S_8\) molecule is represented by the incomplete Lewis diagram above.
(a) The diagram of \(S_8\) shows only bonding pairs of electrons. How many lone pairs of electrons does each S atom in the molecule have?
(b) Based on your answer to part (a), determine the expected value of the S–S–S bond angles in the \(S_8\) molecule.
(c) Write the electron configuration for the S atom in its ground state.
(d) The complete photoelectron spectrum for the element chlorine is represented below. Peak X in the spectrum corresponds to the binding energy of electrons in a certain orbital of chlorine atoms. The electrons in this orbital of chlorine have a binding energy of 273 MJ/mol, while the electrons in the same orbital of sulfur atoms have a binding energy of 239 MJ/mol.
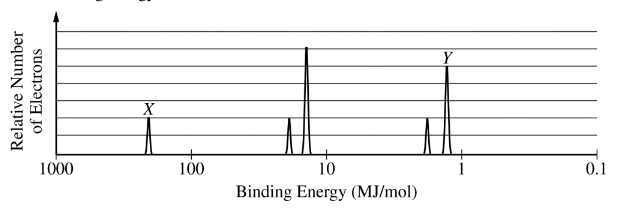
(i) Identify the orbital and explain the difference between the binding energies in terms of Coulombic forces.
(ii) Peak Y corresponds to the electrons in certain orbitals of chlorine atoms. On the spectrum shown, carefully draw the peak that would correspond to the electrons in the same orbitals of sulfur atoms.
\(3S_{8}+8OH^{-}\rightarrow 8S_{3}^{-}+4H00H\)
In an experiment, a student studies the kinetics of the reaction represented above and obtains the data shown in the following table.
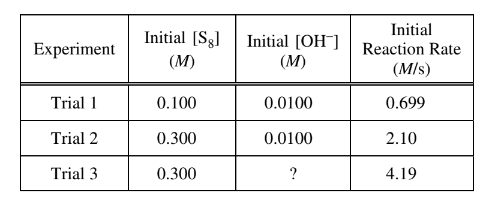
(e) Use the data in the table to do the following.
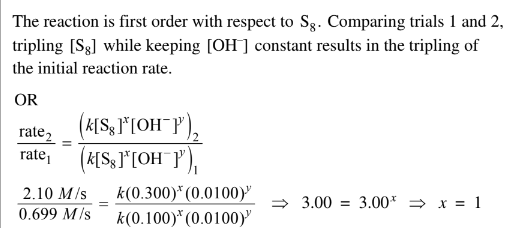
(i) Determine the order of the reaction with respect to \(S_8\) . Justify your answer.
(ii) Determine the value of\( [OH^−]\) that was used in trial 3, considering that the reaction is first order with respect to \(OH^−\). Justify your answer. The next day the student conducts trial 4 using the same concentrations of S 8 and \(OH^−\) as in trial 1, but the reaction occurs at a much slower rate than the reaction in trial 1. The student observes that the temperature in the lab is lower than it was the day before.
(f) Using particle-level reasoning, provide TWO explanations that help to account for the fact that the reaction rate is slower in trial 4.
▶️Answer/Explanation
(a) Two
(b)\(109.5^{\circ}\)
Acceptable range: \(104^{\circ}\leq angle \leq 110^{\circ}\).
(The experimentally determined angle is \(107^{\circ}8\)
(c) \(1s^{2} 2s^{2}2p^63s^2 3p^4\)
OR [Ne]\(3s^23p^4\)
(i) Peak X represents electrons in a 1s orbital. A Cl atom has one more proton in its nucleus than does a S atom; therefore, the electrons in Cl are more strongly attracted to the nucleus, and the binding energy of the 1s electrons in the Cl atom is greater than that of the 1s electrons in the S atom.
(ii) See example of a correct response (dashed peak) above.
(e)(i)
e(ii) Comparing trials 2 and 3, [S8] is kept constant and the initial reaction rate doubles. Since the reaction is first order with respect to\( OH^{-}\), the concentration of \(OH^{-}\) intrial 3 must be\( 2 \times 0.0100 M \)= 0.0200 M.
(f) The temperature was lower on the second day so the average kinetic energy of the reactant particles was lower. Therefore, there were fewer collisions between particles with sufficient
energy to react. Since the temperature was lower, the kinetic energy was lower and the average speed of the particles was lower. At the lower speeds, the reactant particles collided less frequently.
