Question
What mass in grams of hydrogen is produced by the reaction of 4.73 g of magnesium with 1.83 g of water?
\(Mg (s) + 2H_2O (l) \rightarrow Mg(OH)_2 (s) + H_2 (g)\)
A) 0.0485 B) 0.204 C) 0.0162 D) 0.102 E) 0.219
▶️Answer/Explanation
Ans: D
\(Mg + 2H_2O \rightarrow Mg (at)_2 + H_2\) \(0.1946 mol Mg \times \frac{2mol H_2O}{1 mol Mg} = 0.3892 mol H_2O\)
\(4.73g Mg \times \frac{1 mol}{24.31 g} = 0.1946 mol Mg \)ER
\(1.83 gH_2O \times \frac{1 mol}{18.02 g} = 0.1016 mol H_2O \) LR
thus \(H_2O\) is L.R.
0.016 mol \(H_2O \times \frac{1 mol H_2}{2 mol H_2O} \times \frac{2.02 gH_2}{1 mol H_2} = 0.103 g H_2\)
Question
The balanced half-reaction in which dichromate ion is reduced to chromium metal is a __________ process.
A) two-electron
B) six-electron
C) four-electron
D) twelve-electron
E) three-electron
▶️Answer/Explanation
Ans: D
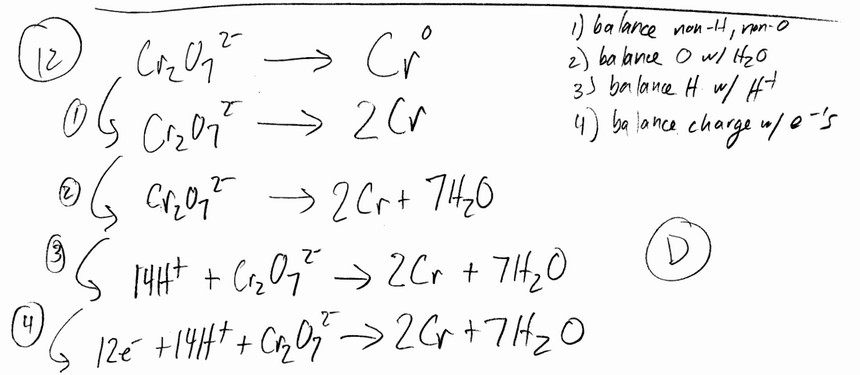
Question
Combining aqueous solutions of \(BaI_2\) and \(K_2SO_4\) affords a precipitate of \(BaSO_4\). Which ion(s) is/are spectator
ions in the reaction?
A) \(Ba^{2+}\) only
B) \(K^+\) and \(I^-\)
C) \(SO_4^{2-}\) and \(I^-\)
D) \(Ba^{2+}\) and \(SO_4^{2-}\)
E) \(K^+\) only
▶️Answer/Explanation
Ans: B
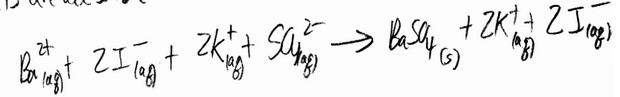
Question
Which equation correctly represents the reaction between carbon dioxide and water?
A) \(CO_2 (aq) + H_2O (l) \rightarrow H_2CO (aq) + O_2 (g)\)
B) \(CO_2 (aq) + H_2O (l) \rightarrow H_2 (g) + CO (g) + O_2 (g)\)
C) \(CO_2 (aq) + H_2O (l) \rightarrow H_2O_2 (aq) + CO (g)\)
D) \(CO_2 (aq) + 2H_2O (l) \rightarrow CH_4 (g) + 2O_2 (aq)\)
E) \(CO_2 (aq) + H_2O (l) \rightarrow H_2CO_3 (aq)\)
▶️Answer/Explanation
Ans: E
