Questions
\(O_{3(g)}+O_{g}\rightarrow 2O_{2(g)}\)
The decomposition of \(O_{3(g)}\) in the upper atmosphere is represented by the equation above. The potential energy diagram for the decomposition of \(O_{3(g)}\) in the presence and absence of \(NO_{(g)}\) is given below.

Which of the following mechanisms for the catalyzed reaction is consistent with the equation and diagram above?
(A) \(2O_{3(g)}+2NO_{g}\rightarrow 4O_{2(g)}+N_{2(g)}\) slow
(B) \(O_{3(g)}+NO_{g}\rightarrow NO_{2(g)}+O_{2(g)}\) slow
\(NO_{2(g)}+ O_{g}\rightarrow NO_{(g)}+O_{2(g)}\) fast
(C) \(NO_{2(g)}+O_{3(g)}\rightarrow NO_{(g)}+2O_{2(g)}\) slow
\(NO_{(g)}+O_{g}\rightarrow NO_{2(g)}\) fast
(D) \(NO_{2(g)}+ O_{g}\rightarrow NO_{3(g)}\) slow
\(NO_{3(g)}+O_{3(g)}\rightarrow NO_{2(g)}+2O_{2(g)}\) fast
▶️Answer/Explanation
Ans: B
Based on the provided potential energy diagram and the given reaction \( \text{O}_3(\text{g}) + \text{O}(\text{g}) \rightarrow 2\text{O}_2(\text{g}) \), the mechanism consistent with the catalytic effect of \( \text{NO}(\text{g}) \) is indeed option B.
The potential energy diagram illustrates that in the presence of \( \text{NO}(\text{g}) \), the activation energy barrier for the reaction is lower compared to its absence. This suggests that \( \text{NO}(\text{g}) \) acts as a catalyst, facilitating the decomposition of \( \text{O}_3(\text{g}) \) by providing an alternative pathway with a lower activation energy.
Option B proposes a two-step mechanism, where the first step is the slow, rate-determining step:
\[ \text{O}_3(\text{g}) + \text{NO}(\text{g}) \rightarrow \text{NO}_2(\text{g}) + \text{O}_2(\text{g}) \quad \text{(slow)} \]
This step involves the reaction of \( \text{O}_3(\text{g}) \) with \( \text{NO}(\text{g}) \) to form \( \text{NO}_2(\text{g}) \) and \( \text{O}_2(\text{g}) \), and it exhibits a lower activation energy barrier in the presence of the catalyst \( \text{NO}(\text{g}) \), as shown in the potential energy diagram.
The second step is a fast step:
\[ \text{NO}_2(\text{g}) + \text{O}(\text{g}) \rightarrow \text{NO}(\text{g}) + \text{O}_2(\text{g}) \quad \text{(fast)} \]
This step regenerates the catalyst \( \text{NO}(\text{g}) \) and produces another \( \text{O}_2(\text{g}) \) molecule, completing the overall reaction \( \text{O}_3(\text{g}) + \text{O}(\text{g}) \rightarrow 2\text{O}_2(\text{g}) \).
Therefore, option B accurately represents the catalytic mechanism consistent with the provided potential energy diagram and the overall reaction equation.
Question
\(2X+Y_2→X_2Y_2\)
A chemist is studying the reaction between the gaseous chemical species X and \(Y_2\), represented by the equation above. Initial rates of reaction are measured at various concentrations of reactants. The results are recorded in the following table.
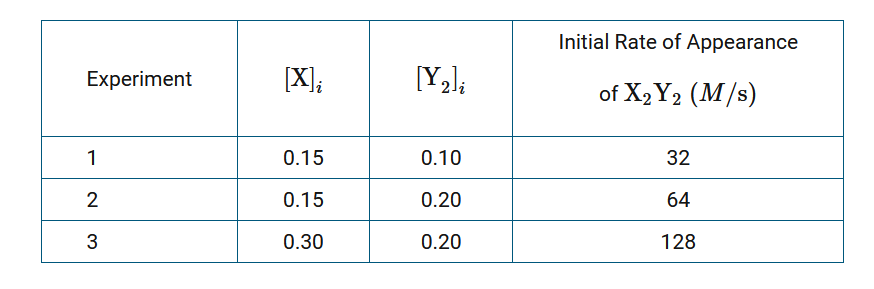
A second chemist repeated the three experiments and observed that the reaction rates were considerably greater than those measured by the first chemist, even though the concentrations of the reactants and the temperature in the laboratory were the same as they were for the first chemist. Which of the following is the best pairing of a claim about a most likely cause for the greater rates measured by the second chemist and a valid justification for that claim?
A The pressures of the gases used by the second chemist must have been lower than those used by the first chemist, thus the collisions between reacting particles were less frequent than they were in the first chemist’s experiments.
B The pressures of the gases used by the second chemist must have been lower than those used by the first chemist, thus the number of collisions with sufficient energy to cause reaction was lower than it was in the first chemist’s experiments.
C The second chemist must have added a catalyst for the reaction, thus providing a different reaction pathway for the reactant particles to react with an activation energy that was lower than that of the uncatalyzed reaction in the first chemist’s experiments.
D The second chemist must have added a catalyst for the reaction, thus providing energy to reactant particles to increase their rate of reaction compared to their rate of reaction in the first chemist’s experiments.
▶️Answer/Explanation
Ans: C
The presence of a catalyst is the most likely cause of the increased rates measured by the second chemist. An alternative reaction pathway with a lower activation energy would explain the greater reaction rates compared to the rates measured by the first chemist.
Question
Step 1: \(HCOOH+H_2SO_4→HCOOH_2^++HSO_4^−\)
Step 2: \(HCOOH_2^+→HCO^++H_2O\)
Step 3: \(HCO^++HSO_4^−→CO+H_2SO_4\)
The elementary steps in a proposed mechanism for the decomposition of \(HCOOH\) are represented above. Which of the following identifies the catalyst in the overall reaction and correctly justifies the choice?
A \(H_2SO_4\) , because it is consumed in the first step of the mechanism and regenerated in a later step.
B \(HCOOH\) , because increasing the concentration of \(HCOOH\) increases the reaction rate.
C \(HCOOH_2^+\), because it is produced in the first step of the mechanism and consumed in a later step.
D \(CO\) , because it is produced in the final step of the reaction mechanism.
▶️Answer/Explanation
Ans:A
The \(H_2SO_4\) is consumed in the first step of the mechanism and regenerated in a later step. This is the definition of a catalyst.
Question
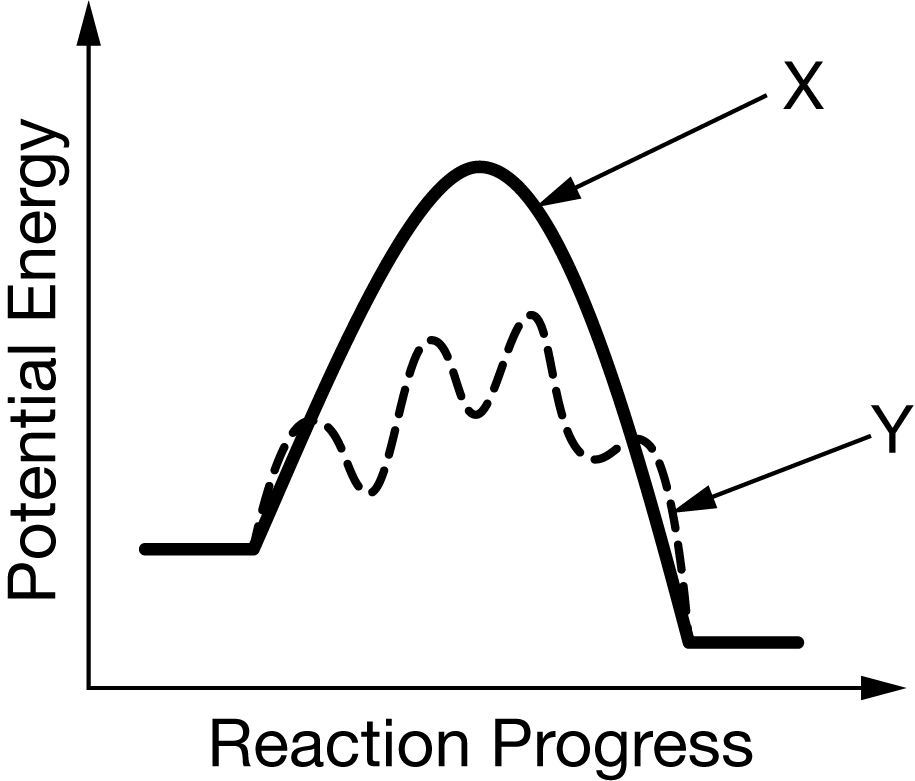
The diagram above shows the reaction energy profiles for a reaction with and without a catalyst. Which of the following identifies the reaction energy profile for the catalyzed reaction, and why?
A Profile X , because a catalyst minimizes the number of elementary steps required for a reaction to proceed.
B Profile X , because the activation energy for the reverse reaction is greater than for the forward reaction, which increases its rate.
C Profile Y , because an increase in the number of transition states increases the rate of the reaction.
D Profile Y , because it introduces a different reaction path that reduces the activation energy.
▶️Answer/Explanation
Ans: D
A catalyst increases the rate of a chemical reaction by either increasing the number of effective collisions or, as in this case, providing a different reaction path with a lower activation energy.
Question
To catalyze a biochemical reaction, an enzyme typically
(A) drives the reaction to completion by consuming byproducts of the reaction
(B) binds temporarily to reactant molecules to lower the activation energy of the reaction
(C) dissociates into additional reactant molecules, thereby increasing the reaction rate
(D) decomposes and releases energy to increase the number of successful collisions between reactant molecules
▶️Answer/Explanation
Ans:B
Question
Of the following, __________ will lower the activation energy for a reaction.
A) increasing the concentrations of reactants
B) adding a catalyst for the reaction
C) raising the temperature of the reaction
D) removing products as the reaction proceeds
E) increasing the pressure
▶️Answer/Explanation
Ans: B