Questions
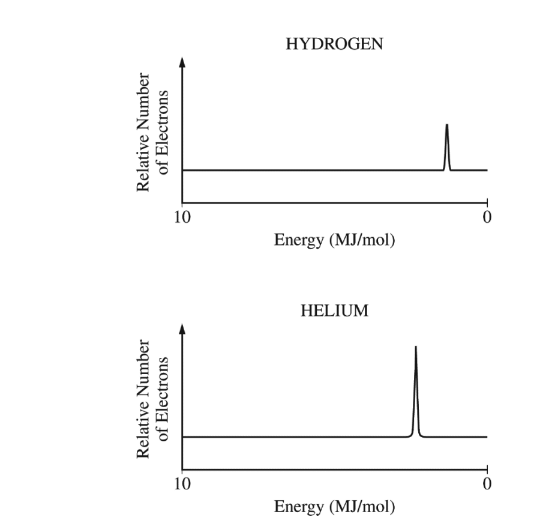
The photoelectron spectra for H and He are represented above. Which of the following statements best accounts for the fact that the peak on the He spectrum is farther to the left and higher than the peak on the H spectrum?
(A) He has an additional valence electron in a higher energy level than the valence electron in H.
(B) He has a greater nuclear charge than H and an additional electron in the same energy level.
(C) He has a completely filled valence shell in which the electrons are a greater distance from the nucleus
than the distance between the H nucleus and its electron.
(D) It takes longer for the electrons in He to be removed due to the higher nuclear mass of He.
▶️Answer/Explanation
Ans: B
The correct answer is (B) He has a greater nuclear charge than H and an additional electron in the same energy level. This accounts for the fact that more energy is required to remove an electron from helium, resulting in a peak that is farther to the left (indicating higher ionization energy) and higher (indicating more electrons are being removed at this higher energy).
Helium (He) has two protons in its nucleus, giving it a greater nuclear charge than hydrogen (H), which has only one proton. The increased nuclear charge exerts a stronger attractive force on the electrons, making them more difficult to remove and thus requiring more energy to ionize. Additionally, helium has two electrons in the same principal energy level (1s), while hydrogen has only one. The presence of two electrons in helium also contributes to a higher peak in the photoelectron spectrum, as there are more electrons at that energy level to be removed compared to hydrogen. This explains the differences observed in the spectra for H and He
Questions

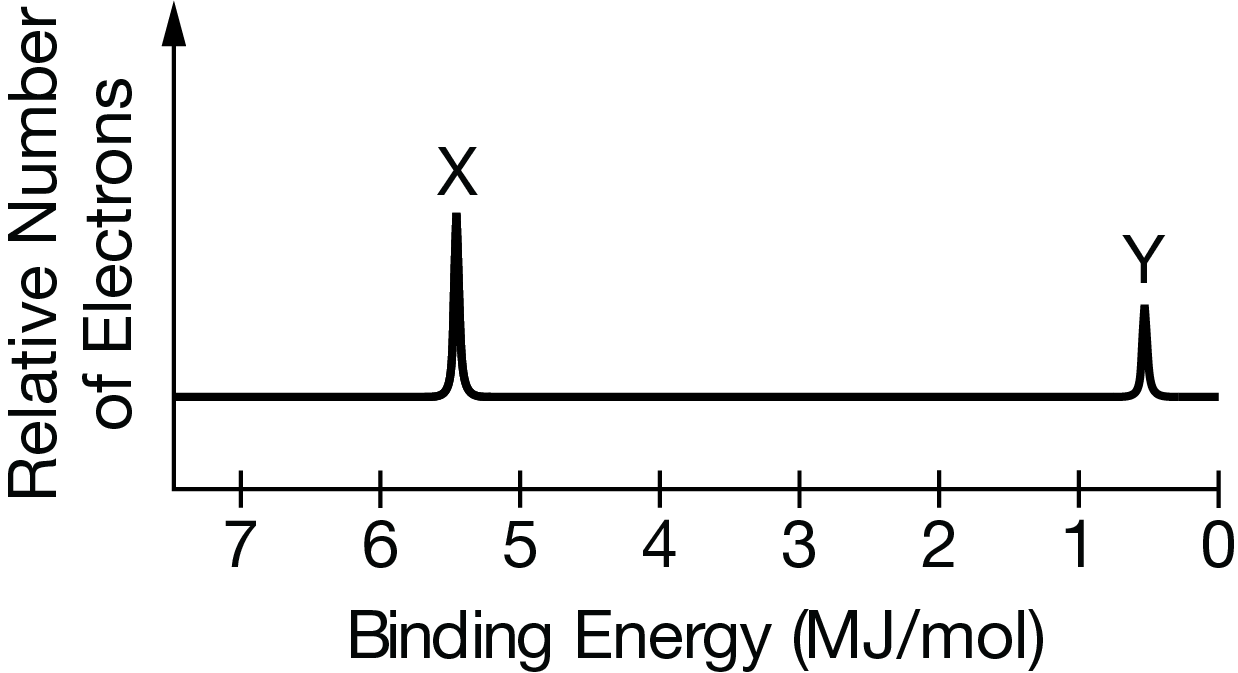
The complete photoelectron spectra of neutral atoms of two unknown elements, X and Y, are shown above. Which of the following can be inferred from the data?
(A) Element X has a greater electronegativity than element Y does.
(B) Element X has a greater ionization energy than element Y does.
(C) Element Y has a greater nuclear charge than element X does.
(D) The isotopes of element Y are approximately equal in abundance, but those of element X are not.
▶️Answer/Explanation
Ans: C
The reason is that the photoelectron spectrum shows the binding energies of electrons in different atomic orbitals. These binding energies are directly proportional to the effective nuclear charge experienced by the electrons.
From the spectra, we can see that for all orbital peaks, the binding energies are higher for element Y compared to element X. This indicates the electrons in element Y experience a stronger effective nuclear charge and are more tightly bound.
A greater effective nuclear charge implies a higher nuclear charge in the nucleus for element Y versus element X.
Therefore, based on the provided data, the appropriate inference is that element Y has a greater nuclear charge than element X.
Question
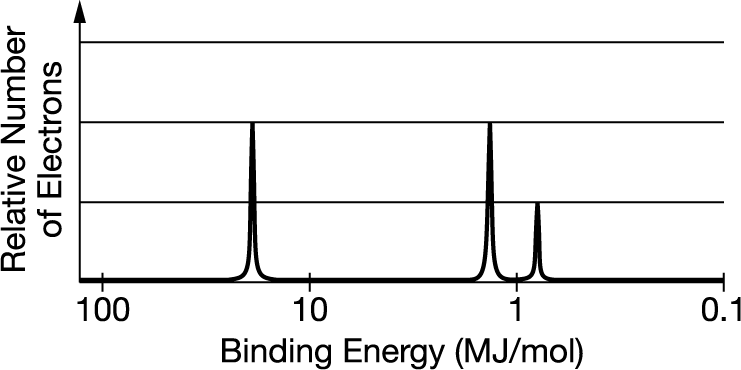
The complete photoelectron spectrum of an element is given above. Which labeled peak corresponds to the is electrons and why?
▶️Answer/Explanation
Ans: B Greater binding energies correlate to stronger attractions to the nucleus.
Question
The photoelectron spectrum for the element boron is represented above. Which of the following best explains how the spectrum is consistent with the electron shell model of the atom?
A The spectrum shows an odd number electrons.
B The spectrum shows a single electron in the 2p subshell.
C The spectrum shows equal numbers of electrons in the first and second electron shells.
D The spectrum shows three electrons with the same binding energy in the second electron shell.
▶️Answer/Explanation
Ans:B The spectrum is consistent with the electron configuration for boron: \(1s^22^s2^2p^1\). The leftmost peak represents the two electrons in the filled 1s subshell. The two peaks on the right represent the two electrons in the filled 2s subshell and the single electron in the 2p subshell.
Question

The complete photoelectron spectrum of the element carbon is represented above. Which of the following best explains how the spectrum is consistent with the electron shell model of the atom?
A The spectrum shows four electrons in the inner electron shell.
B The spectrum shows equal numbers of electrons in the three occupied electron subshells.
C The spectrum shows that all the electrons in the valence shell have the same binding energy.
▶️Answer/Explanation
Ans: B The peak on the left represents two 1s electrons, and the two peaks on the right represent two 2s electrons and two 2p electrons, respectively.
Question
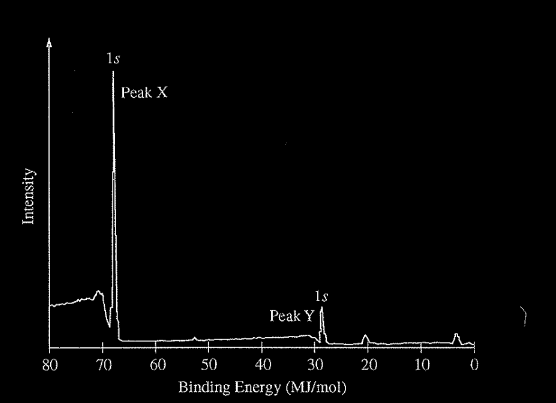
A sample containing atoms of C and F was analyzed using x-ray photoelectron spectroscopy. The portion of the spectrum showing the Is peaks for atoms of the two elements is shown above. Which of the following correctly identifies the 1s peak for the F atoms and provides an appropriate explanation?
(A) Peak X, because F has a smaller first ionization energy than C has.
(B) Peak X, because F has a greater nuclear charge than C has.
(C) Peak Y, because F is more electronegative than C is.
(D) Peak Y, because F has a smaller atomic radius than Ĉ has.
▶️Answer/Explanation
Ans:B
Question
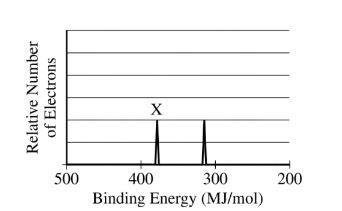
The photoelectron spectra of the 1s electrons of two isoelectronic species, \(Ca^{2+}\) and Ar, are shown above. Which of the following correctly identifies the species associated with peak X and provides a valid justification?
(A) Ar, because it has completely filled energy levels
(B) Ar, because its radius is smaller than the radius of \(Ca^{2+}\)
(C) \(Ca^{2+}\), because its nuclear mass is greater than that of Ar
(D)\( Ca^{2+}\), because its nucleus has two more protons than the nucleus of Ar has
▶️Answer/Explanation
Ans:C