Question

The masses of carbon and hydrogen in samples of four pure hydrocarbons are given above. The hydrocarbon in which sample has the same empirical formula as propene,\( C_{3}H_{6}\) ?
(A) Sample A
(B) Sample B
(C) Sample C
(D) Sample D
▶️Answer/Explanation
Ans:B
To determine which sample has the same empirical formula as propene (\(C_3H_6\)), we need to find a sample with a ratio of carbon to hydrogen atoms that matches the ratio in propene.
The empirical formula of propene (\(C_3H_6\)) tells us that the ratio of carbon atoms to hydrogen atoms is 1:2. We need to find a sample where the ratio of carbon to hydrogen atoms matches this.
Let’s calculate the ratios for each sample:
Sample A: \(60 \, \text{g C} : 12 \, \text{g H} = 5 : 1\)
Sample B: \(72 \, \text{g C} : 12 \, \text{g H} = 6 : 1\)
Sample C: \(84 \, \text{g C} : 10 \, \text{g H} = 8.4 : 1\)
Sample D: \(90 \, \text{g C} : 10 \, \text{g H} = 9 : 1\)
Among these, the only sample with a ratio close to 1:2 is Sample B. Its ratio is 6:1, which can be reduced to 3:1, which matches the 1:2 ratio needed for propene. So, the correct answer is:(B) Sample B
Questions
A sample of a solid labeled as NaCl may be impure. A student analyzes the sample and determines that it contains 75 percent chlorine by mass. Pure \(NaCl_{(s)}\) contains 61 percent chlorine by mass. Which of the following statements is consistent with the data?
(A) The sample contains only \(NaCl_{(s)}\).
(B) The sample contains \(NaCl_{(s)}\) and \(NaI_{(s)}\).
(C) The sample contains \(NaCl_{(s)}\) and \(KCl_{(s)}\).
(D) The sample contains \(NaCl_{(s)}\) and \(LiCl_{(s)}\).
▶️Answer/Explanation
Ans: D
In this question,
The pure \(\mathrm{NaCl}\) (s) contains \(61\%\) chlorine but the impure sample contains \(75 \%\) chlorine by mass.
This can be possible when sample contains \(\mathrm{LiCl}\). Because when \(\mathrm{LiCl}\) is added then the mass of chlorine added is more and hence the percent of chlorine will be increases from 61 to 75 percent. Therefore, the sample contains \(\mathrm{NaCl}\) (s) and \(\mathrm{LiCl}(\mathrm{s})\).
option (D) The sample contains \(\mathrm{NaCl}_{(s)}\) and \(\mathrm{LiCl}\) (s)
Question
\(Ba^{2+}(aq)+SO_{4}^{2-}(aq)\rightarrow BaSO_{4}(s)\)
A student obtains a 10.0g sample of a white powder labeled as \(BaCl_{2}\). After completely dissolving the powder in 50.0mL of distilled water, the student adds excess \(Na_{2}SO_{4}(s)\), which causes a precipitate of \(BaSO_{4}(s)\) to form, as represented by the equation above. The student filters the \(BaSO_{4}(s)\), rinses it, and dries it until its mass is constant. Which of the following scientific questions could best be answered based on the results of the experiment?
A Is the \(Na_{2}SO_{4}(s)\) used in the experiment pure?
B Is the \(BaCl_{2}(s)\) used in the experiment pure?
C What is the molar solubility of \(BaCl_{2}\) in water?
D What is the molar solubility of \(BaSO_{4}\) in water?
▶️Answer/Explanation
Ans:B
The calculated number of moles of Ba in the precipitate can be compared with the expected number of moles of Ba in 10.0g of \(BaCl_{2}\). The presence of fewer moles of in the precipitate would imply that the \(BaCl_{2}\) was not pure.
Question
A student measures the mass of a sample of a metallic element, M. Then the student heats the sample in air, where it completely reacts to form the compound MO. The student measures the mass of the compound that was formed. Which of the following questions can be answered from the results of the experiment?
A What is the density of M?
B What is the molar mass of M?
C What is the melting point of M?
D What is the melting point of MO?
▶️Answer/Explanation
Ans:B
Correct. The number of moles of O in the compound is equal to the number of moles of M and can be calculated using the difference in the masses of the sample and the compound. The molar mass of M is calculated by dividing the mass of the sample of M by the number of moles of M.
Question
A 42.0gsample of compound containing only C and H was analyzed. The results showed that the sample contained 36.0g of C and 6.0g of H. Which of the following questions about the compound can be answered using the results of the analysis?
A What was the volume of the sample?
D What is the empirical formula of the compound?
▶️Answer/Explanation
Ans:C
The results do not include any information about the chemical stability of the compound. The number of moles of C and H is calculated by dividing the masses of each element in the compound and their respective molar masses. The ratio of the number of moles of C to H can be used to determine the empirical formula of the compound.
Question
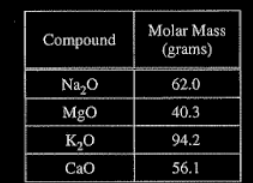
According to the information in the table above, a 1.00 g sample of which of the following contains the greatest mass of oxygen?
(A) \(Na_{2}O\)
(B) MgO
(C) \(K_{2}O\)
(D) CO
▶️Answer/Explanation
Ans:B
Question
A 23.0 g sample of a compound contains 12.0 g of C, 3.0 g of H, and 8.0 g of O. Which of the following is the empirical formula of the
compound?
(A) \(CH_{3}O\)
(B) \(C_{2}H_{6}\)O
(C)\( C_{3}H_{9}O_{2}\)
(D) \( C_{4}H_{12}O_{2}\)
▶️Answer/Explanation
Ans:C