Question
A student combines a solution of \(NaCl\)(aq) with a solution of \(AgNO_3\)(aq), and a precipitate forms.
Assume that 50.0mL of 1.0M \(NaCl\)(aq) and 50.0mL of 1.0M \(AgNO_3\)(aq) were combined. According to the balanced equation, if 50.0mL of 2.0M NaCl(aq) and 50.0mL of 1.0M \(AgNO_3\) were combined, the amount of precipitate formed would
A double, because all of the coefficients are 1
B double, because the amount of one of the reactants was doubled
C not change, because all of the coefficients are 1
D not change, because the amount of \(AgNO_3\)(aq) did not change
▶️Answer/Explanation
Ans: D
In the equation \(Ag^+(aq)+Cl^−(aq)→AgCl(s)\), all the coefficients are 1. Therefore, NaCl(aq) and \(AgNO_3\)(aq) will combine in equal moles to produce an equal number of moles of AgCl(s) in both trials, regardless if the moles of \(NaCl\)(aq) are doubled. The quantity of \(AgNO_3\)(aq) will limit the amount of precipitate that can form in the second trial.
Question
\(HC_2H_3O_2(aq)+OH^−(aq)→C_2H_3O_2^−(aq)+H_2O(l)\)
A student carried out a titration using \(HC_2H_3O_2(aq)\) and \(NaOH(aq)\) . The net ionic equation for the neutralization reaction that occurs during the titration is represented above. The \(NaOH(aq)\) was added from a buret to the \(HC_2H_3O_2(aq)\) in a flask. The equivalence point was reached when a total of 20.0mL of \(NaOH(aq)\) had been added to the flask. How does the amount of \(HC_2H_3O_2(aq)\) in the flask after the addition of 5.0mL of NaOH(aq) compare to the amount of \(HC_2H_3O_2(aq)\) in the flask after the addition of 1.0mL of \(NaOH(aq)\) , and what is the reason for this result?
A It is less because more \(HC_2H_3O_2(aq)\) reacted with the base.
B It is the same because the equivalence point has not been reached.
C It is the same because all of the coefficients in the neutralization equation are 1.
D It is greater because \(HC_2H_3O_2(aq)\) is a proton donor.
▶️Answer/Explanation
Ans:A
During the titration, \(NaOH(aq)\) is added to the contents of the flask, where \(HC_2H_3O_2(aq)\) reacts with \(OH^−(aq)\) ions in a 1 to 1 ratio. As the titration proceeds, more and more \(HC_2H_3O_2(aq)\)reacts until the titration reaches the equivalence point, when all the \(HC_2H_3O_2(aq)\) has reacted.
Question
\(K_2SO_3(aq)+2HNO_3(aq)→2KNO_3(aq)+SO_2(g)+H_2O(l)\)
According to the balanced chemical equation above, when 100.0mL of 0.100M \(K_2SO_3(aq)\) is mixed with 100.0mL of 0.200M \(HNO_3(aq)\) at 30°C and 1 atm, the volume of \(SO_2\) gas produced is 0.24 L. If it is assumed that the reaction goes to completion, which of the following changes would double the volume of \(SO_2\) produced at the same temperature and pressure, and why? (For each change, assume that the other solutions and volumes remain the same.)
A Using 200.0mL of the 0.100M \(K_2SO_3(aq) \) , because it then becomes the reactant in excess
B Using 200.0mL of the 0.200MHNO3(aq), because the volume of \(SO_2\) produced is inversely proportional to the number of moles at constant temperature and pressure
C Using 200.0mL of 0.100M \(K_2SO_3(aq) \) and 200.0mL of 0.200M \(HNO_3(aq)\) , because this provides double the number of moles with the correct stoichiometric ratio
D Using 400.0mL of 0.100M \(K_2SO_3(aq) \) and 200.0mL of 0.200M \(HNO_3(aq)\) , because this provides the same number of moles of each reactant
▶️Answer/Explanation
Ans: C
At constant temperature and pressure, the volume of \(SO_2\) produced is directly proportional to the number of moles formed from the reaction. Because of the 1:2 mole ratio between \(K_2SO_3 \) and \(HNO_3\) in the balanced equation, the stoichiometric amounts required to double the number of moles of \(SO_2\) should preserve that ratio. Since the reaction was initially done with the correct stoichiometric amounts, the number of moles of each reactant must be doubled, which is accomplished when volume of each of the initial solutions is doubled.
Question
\(MnO_{4}^{-}+5Fe^{2+}+8H^{+}\rightarrow Mn^{2+}+5Fe^{3+}+4H_{2}O\)
In the reaction represented above, the number of \(MnO_{4}^{-}\) ions that react must be equal to which of the following?
(A) One-fifth the number of \(Fe^{2+}\) ions that are consumed
(B) Eight times the number of\( H^{+}\) ions that are consumed
(C) Five times the number of\( Fe^{3+}\) ions that are produced
(D) One-half the number of \(H_{2}O \)molecules that are produced
▶️Answer/Explanation
Ans:A
Question
When water is added to a mixture of Na_{2}O_{2}(s) and S(s), a redox reaction occurs, as represented by the
equation below.
\(2 Na_{2}O_{2}(s) + S(s) + 2 H_{2}O(l) \)\rightarrow 4 NaOH(aq) + SO_{2}(aq)\) \(\Delta H^{\circ}_{298}=-610kj/mol_{rxn}; \Delta S^{\circ}=-7.3j/(k.mol_{rxn})\)
Two trials are run, using excess water. In the first trial, 7.8 g of \(Na_2O_2(s)\) (molar mass 78 g/mol) is mixed with 3.2 g of S(s). In the second trial, 7.8 g of \(Na_2O_2(s)\) is mixed with 6.4 g of S(s). The \(Na_2O_2(s)\) and S(s) react as completely as possible. Both trials yield the same amount of \(SO_2\)(aq). Which of the following identifies the limiting reactant and the heat released, q, for the two trials at 298 K?
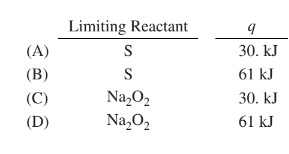
▶️Answer/Explanation
Ans:D
- adminA Level Biology- Exam Style Practice Questions with Answer-Topic Wise-Paper 112 WeeksAll levels24 Lessons0 Quizzes6315 Students$11.00
- adminA Level Biology- Exam Style Practice Questions with Answer-Topic Wise-Paper 212 WeeksAll levels24 Lessons0 Quizzes6316 Students$11.00
- adminA Level Biology- Exam Style Practice Questions with Answer-Topic Wise-Paper 412 WeeksAll levels20 Lessons0 Quizzes6315 Students$11.00
- adminA Level Chemistry- Exam Style Practice Questions with Answer-Topic Wise-Paper 112 WeeksAll levels51 Lessons0 Quizzes6312 Students$11.00
- adminA Level Chemistry- Exam Style Practice Questions with Answer-Topic Wise-Paper 212 WeeksAll levels51 Lessons0 Quizzes6312 Students$11.00