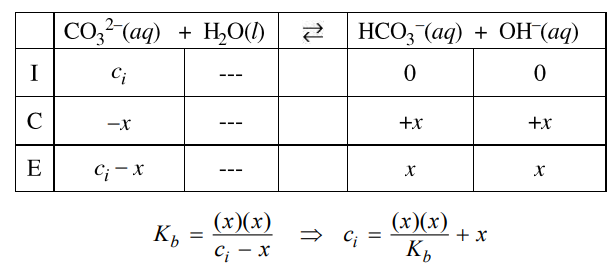Question
A student is given 50.0 mL of a solution of Na2CO3 of unknown concentration. To determine the concentration of the solution, the student mixes the solution with excess 1.0 M Ca(NO3)2(aq), causing a precipitate to form.
The balanced equation for the reaction is shown below.
Na2CO3(aq) + Ca(NO3)2(aq) → 2 NaNO3(aq) + CaCO3(s)
(a) Write the net ionic equation for the reaction that occurs when the solutions of Na2CO3 and Ca(NO3)2 are mixed.
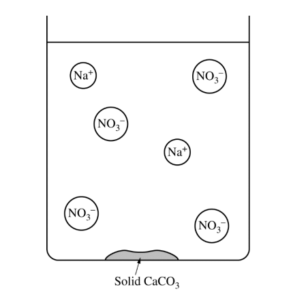
(b) The diagram below is incomplete. Draw in the species needed to accurately represent the major ionic species remaining in the solution after the reaction has been completed.
The student filters and dries the precipitate of CaCO3 (molar mass 100.1 g/mol) and records the data in the table below.

(c) Determine the number of moles of Na2CO3 in the original 50.0 mL of solution.
(d) The student realizes that the precipitate was not completely dried and claims that as a result, the calculated Na2CO3 molarity is too low. Do you agree with the student’s claim? Justify your answer.
(e) After the precipitate forms and is filtered, the liquid that passed through the filter is tested to see if it can conduct electricity. What would be observed? Justify your answer.
The student decides to determine the molarity of the same Na2CO3 solution using a second method. When
Na2CO3 is dissolved in water, CO32−(aq) hydrolyzes to form HCO3−(aq), as shown by the following equation.
CO32−(aq) + H2O(l) ⇔ HCO3−(aq) + OH−(aq) \(K_{b}=\frac{[HC{O_{3}}^{-}][OH^{-}]}{[{CO_{3}}^{2-}]}=2.1\times 10^{-4}\)
(f) The student decides to first determine [OH−] in the solution, then use that result to calculate the initial concentration of CO32−(aq).
(i) Identify a laboratory method (not titration) that the student could use to collect data to determine [OH−] in the solution.
(ii) Explain how the student could use the measured value in part (f)(i) to calculate the initial concentration of CO32−(aq). (Do not do any numerical calculations.)
(g) In the original Na2CO3 solution at equilibrium, is the concentration of HCO3−(aq) greater than, less than, or equal to the concentration of CO32−(aq) ? Justify your answer.
(h) The student needs to make a CO32−/HCO3− buffer. Is the Na2CO3 solution suitable for making a buffer with a pH of 6? Explain why or why not.
▶️Answer/Explanation
Ans:
(a)
| Ca2+(aq) + CO32-(aq) → CaCO3(s) |
(b)
| The drawing shows one Ca2+ ion. |
(c)
\(0.93 g CaCO_{3}\times \frac{1 mol CaCO_{3}}{100.1 g}=0.0093 mol CaCO_{3}\) \(0.0093 mol CaCO_{3}\times \frac{1 mol Na_{2}CO_{3}}{1 molCaCO_{3} }=0.0093 mol Na_{2}CO_{3}\) |
(d)
| Disagree. The presence of water in the solid will cause the measured mass of the precipitate to be greater than the actual mass of CaCO3. As a result, the calculated number of moles of CaCO3 and moles of Na2CO3 will be greater than the actual moles present. Therefore the calculated concentration of Na2CO3(aq) will be too high. |
(e)
| The liquid conducts electricity because ions (Na+(aq), Ca2+(aq), and NO3–(aq) are present in the solution. |
(f) (i)
| Determine the pH of the solution using a pH meter. |
(ii)
First determine [OH–] using pOH = 14 – pH, then [OH–] = 10-pOH. Then, use the Kb expression and an ICE table (see example below to determine [CO32-] and [HCO3–] at equilibrium. The initial concentration of CO32-, ci, is equal to the sum of the equilibrium concentrations of CO32- and HCO3–.
|
(g)
| Less than. The small value of Kb, 2.1 × 10-4, indicates that the reactants are favored. |
(h)
| No, the Na2CO3 solution is not suitable. The pKa of HCO3– is 10.32. Buffers are effective when the required pH is approximately equal to the pKa of the weak acid. An acid with a pKa of 10.32 is not appropriate to prepare a buffer with a pH of 6. |
Question
| Mass of KI tablet | 0.425 g |
| Mass of thoroughly dried filter paper | 1.462 g |
| Mass of filter paper + precipitate after first drying | 1.775 g |
| Mass of filter paper + precipitate after second drying | 1.699 g |
| Mass of filter paper + precipitate after third drying | 1.698 g |
A student is given the task of determining the I− content of tablets that contain KI and an inert, water-soluble sugar as a filler. A tablet is dissolved in 50.0 mL of distilled water, and an excess of 0.20 M Pb(NO3)2(aq) is added to the solution. A yellow precipitate forms, which is then filtered, washed, and dried. The data from the experiment are shown in the table above.
(a) For the chemical reaction that occurs when the precipitate forms,
(i) write a balanced, net-ionic equation for the reaction, and
(ii) explain why the reaction is best represented by a net-ionic equation.
(b) Explain the purpose of drying and weighing the filter paper with the precipitate three times.
(c) In the filtrate solution, is [K+] greater than, less than, or equal to [NO3−] ? Justify your answer.
(d) Calculate the number of moles of precipitate that is produced in the experiment.
(e) Calculate the mass percent of I− in the tablet.
(f) In another trial, the student dissolves a tablet in 55.0 mL of water instead of 50.0 mL of water. Predict whether the experimentally determined mass percent of I− will be greater than, less than, or equal to the amount calculated in part (e). Justify your answer.
(g) A student in another lab also wants to determine the I− content of a KI tablet but does not have access to Pb(NO3)2 . However, the student does have access to 0.20 M AgNO3 , which reacts with I−(aq) to produce AgI(s). The value of Ksp for AgI is 8.5 × 10−17.
(i) Will the substitution of AgNO3 for Pb(NO3)2 result in the precipitation of the I− ion from solution? Justify your answer.
(ii) The student only has access to one KI tablet and a balance that can measure to the nearest 0.01 g.
Will the student be able to determine the mass of AgI produced to three significant figures? Justify your answer.
▶️Answer/Explanation
Ans:
(a) (i)
| Pb2+ + 2 I– → PbI2 |
(ii)
| The net-ionic equation shows the formation of the PbI2 (s) from Pb2+(aq) and I–(aq) ions, omitting the non-reacting species (spectator ions), K+(aq) and NO3 – (aq). |
(b)
| The filter paper and precipitate must be dried several times (to a constant mass) to ensure that all the water has been driven off. |
(c)
| [K+] is less than [NO3– ] because the source of the NO3–, the 0.20 M Pb(NO3)2 (aq), was added in excess. |
(d)
1.698 g – 1.462 g = 0.236 g PbI2(s) 0.236 g PbI2 × \(\frac{1 mol PbI_{2}}{461.0 g PbI_{2}}=5.12\times 10^{-4}mol PbI_{2}\) |
(e)
\(5.12 \times 10^{-4}mol PbI_{2}\times \frac{2 mol I^{-}}{1 mol PbI_{2}}=1.02\times 10^{-3}mol I^{-}\) \(1.02 \times 10^{-3}mol I^{-}\times \frac{126.91 g I^{-}}{1 mol I^{-}}=0.130 g\times I^{-} \) in one tablet \(\frac{0.130 g I^{-}}{0.425 g KI tablet}=0.306 = 30.6% I^{-} \) per KI tablet |
(f)
| The mass percent of I– will be the same. Pb2+(aq) was added in excess, ensuring that essentially no I– remained in solution. The additional water is removed by filtration and drying, leaving the same mass of dried precipitate. |
(g) (i)
| Yes. Addition of an excess of 0.20 M AgNO3(aq) will precipitate all of the I– ion present in the solution because AgI is insoluble, as evidenced by its low value of Ksp . |
(ii)
| No. If masses can be measured to ±0.01 g, then the mass of the dry AgI(s) precipitate (which is less than 1 g) will be known to only two significant figures. |
Question
Discuss the following phenomena in terms of the chemical and physical properties of the substances involved and general principles of chemical and physical change.
(a) As the system shown on the right approaches equilibrium, what change occurs to the volume of water in beaker A ? What happens to the concentration of the sugar solution in beaker B ? Explain why these changes occur. 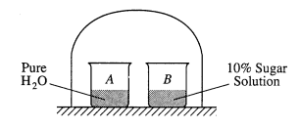
(b) A bell jar connected to a vacuum pump is shown on the right. As the air pressure under the bell jar decreases, what behavior of water in the beaker will be observed? Explain why this occurs. 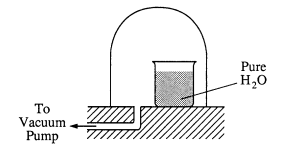
(c) What will be observed on the surfaces of zinc and silver strips shortly after they are placed in separate solutions of CuSO4, as shown on the right? Account for these observations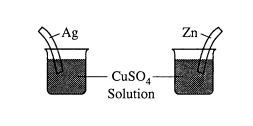
(d) A water solution of I2 is shaken with an equal volume of a nonpolar solvent such as TTE (trichlorotrifluoroethane). Describe the appearance of this system after shaking. (A diagram may be helpful.) Account for this observation 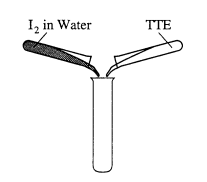
▶️Answer/Explanation
Ans:
(a) Volume decreases in beaker A; the concentration decreases in beaker B (either observation earns 1 point provided other one is not wrong)
The vapor pressure of pure H2O is greater than the vapor pressure of H2O in solution,
OR,
the rate of evaporation of H2O molecules from pure H2O is greater than that from the sugar solution, while the condensation rates are the same.
(b) The water will begin to boil (or evaporate).
The external pressure on the water will become equal to the vapor pressure of the water, causing it to boil,
OR,
the drop in external pressure causes the boiling point to drop to the temperature of the water.
(c) Solid copper is deposited on the zinc strip; the zinc strip goes into solution. No reaction occurs with silver.
Zinc is a better reducing agent or a more active metal than copper and will be oxidized. Silver is a less reactive metal than copper is.
(d) Two layers will form, one of which is colored. Iodine is nonpolar and will dissolve in TTE. Water-is polar and will not dissolve in TTE.
Note: placement of I2 must be correctly indicated for 2nd point.