Question
\(2 NO_2(g) → N_2O_4\) (g)
dark brown colorless
The dimerization of NO2(g), an exothermic process, is represented by the equation above.
Which of the following experimental techniques will allow the most accurate determination of the concentration of \(NO_2\ (g) at equilibrium?
(A) Paper chromatography
(B) Gravimetric analysis
(C) Titration
(D)Spectrophotometry
▶️Answer/Explanation
Ans:D
To accurately determine the concentration of \(NO_2(g)\) at equilibrium, a technique that directly measures the concentration of gases would be most suitable. Among the options provided:
(A) Paper chromatography: This technique is primarily used for separating and analyzing components of a mixture based on their affinity to a stationary phase and mobile phase. It is not suitable for directly measuring the concentration of gases.
(B) Gravimetric analysis: Gravimetric analysis involves the determination of the quantity of a substance by weighing the sample before and after a chemical reaction. While it can be used for certain types of reactions, it may not be the most practical method for analyzing gas-phase reactions.
(C) Titration: Titration involves the gradual addition of a solution of known concentration (titrant) to a solution of unknown concentration (analyte) until the reaction between the two is complete. While titration can be used to determine the concentration of some gases when they react with a suitable titrant, it may not be the most accurate method for determining the concentration of \(NO_2(g)\) directly.
(D) Spectrophotometry: Spectrophotometry involves the measurement of the absorption or emission of electromagnetic radiation by substances. In the case of gases, spectrophotometry can be used to measure the absorbance of light by the gas at specific wavelengths. Since \(NO_2(g)\) has characteristic absorption bands in the visible or ultraviolet regions of the spectrum, spectrophotometry can accurately determine its concentration by measuring the absorbance of light by the gas.
Therefore, the most accurate technique for determining the concentration of \(NO_2(g)\) at equilibrium is: (D) Spectrophotometry
Question
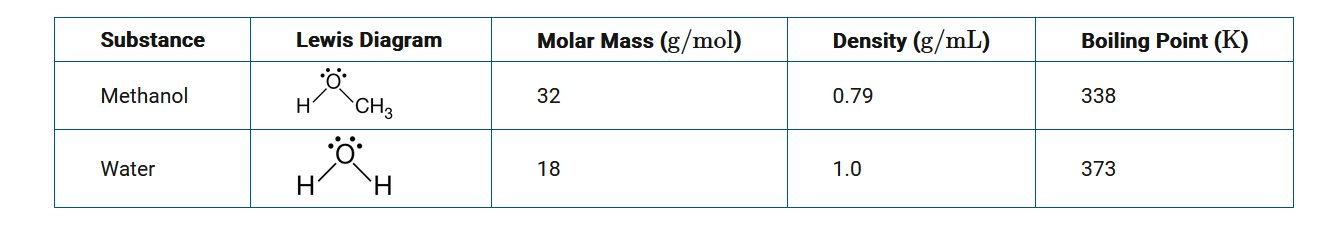
When methanol and water are mixed together, they form a homogeneous mixture. Based on the information in the table above, which of the following would be the best procedure for separating a mixture of methanol and water?
A Filtration
B Distillation
C Paper chromatography
▶️Answer/Explanation
Ans:B
Distillation is a separation technique that takes advantage of differences in boiling points. Methanol has a lower boiling point than water and can be separated from water using a distillation apparatus.
Question
Which of the following methods is most appropriate to use to determine the number of different-colored components in a sample of black ink?
A Distillation at atmospheric pressure
B Elemental analysis to determine the mass ratio of C:H:N:O
C Column chromatography using a nonpolar stationary phase and water as the mobile phase
▶️Answer/Explanation
Ans: D
Paper chromatography is appropriate to use to determine the number of components, because it can be used to separate very small quantities of a mixture into its components. Trying different solutions with a range of polarities as the mobile phase is helpful for identifying the solution that results in the greatest difference in retention factors among the components.
Question
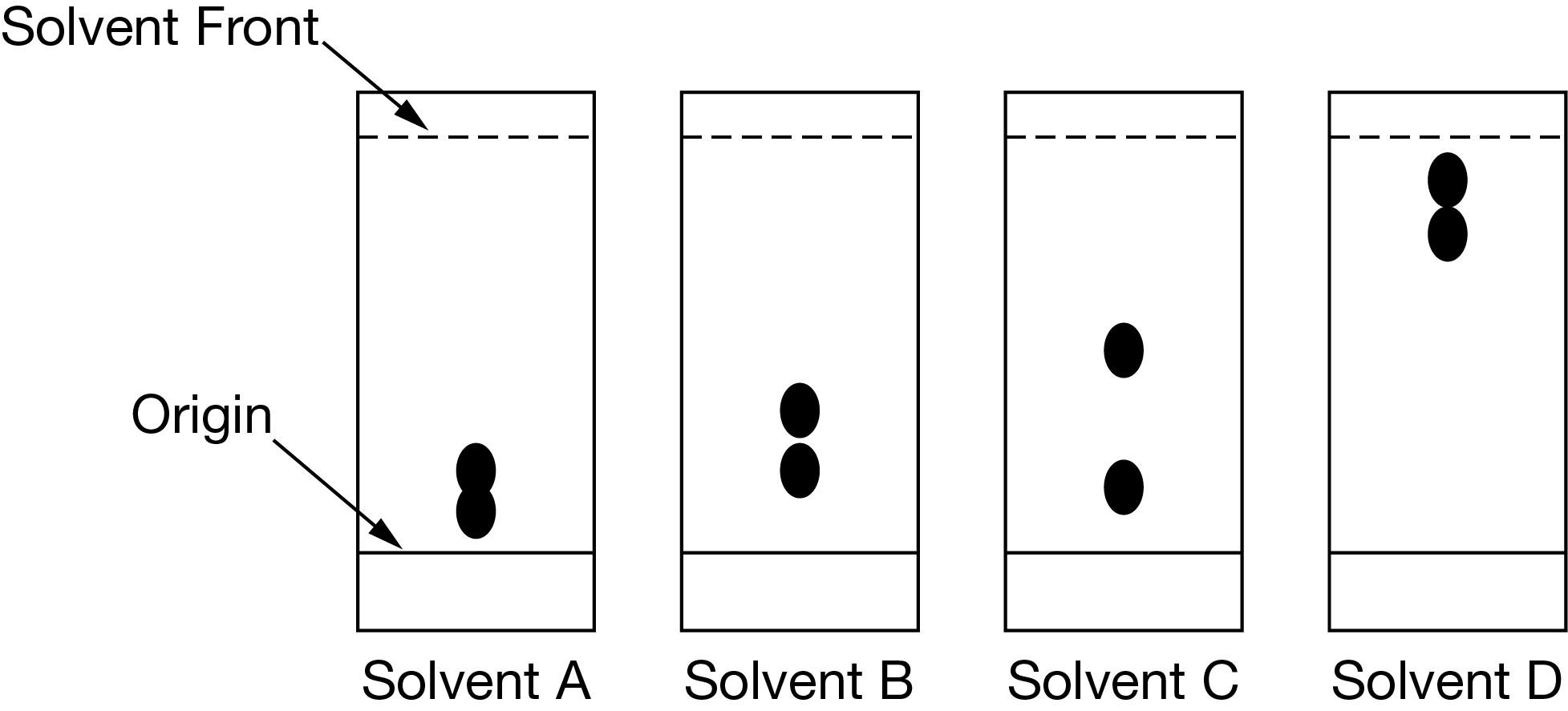
The diagram above shows thin-layer chromatograms of the same mixture of two compounds. Based on the chromatograms, which solvent would be most effective at separating the two compounds if the same stationary phase is used for column chromatography?
▶️Answer/Explanation
Ans: C
For the two compounds in the mixture, the difference in affinity for solvent C is the largest of the four solvents. Therefore, the most effective separation of the compounds can be achieved in a column by using solvent C as the mobile phase.
Question
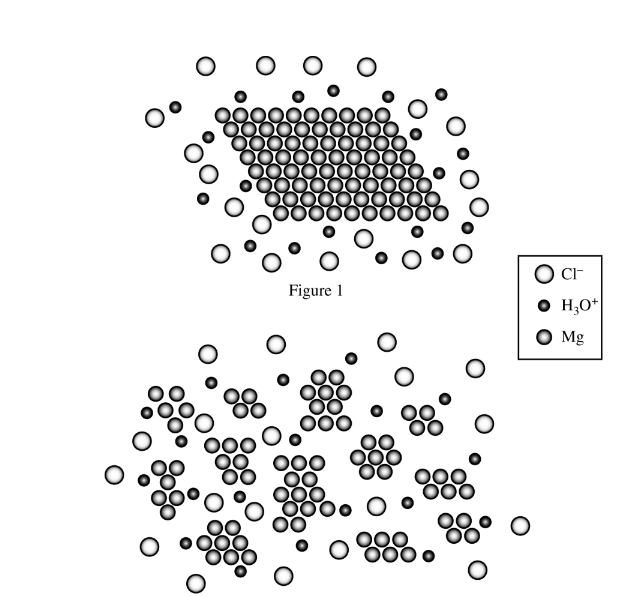
Two samples of Mg(s) of equal mass were placed in equal amounts of HCl(aq) contained in two separate reaction vessels. Particle representations of the mixing of Mg(s) and HCl(aq) in the two reaction vessels are shown in Figure 1 and Figure 2 above. Water molecules are not included in the particle representations. Which of the reactions will initially proceed faster, and why?
(A) The reaction in Figure 1, because the atoms of Mg are more concentrated than those in Figure 2
(B) The reaction in Figure 1, because the Mg(s) in Figure 1 has a larger mass than the Mg(s) in Figure 2
(C) The reaction in Figure 2, because more Mg atoms are exposed to HCl(aq) in Figure 2 than in Figure 1
(D) The reaction in Figure 2, because the Mg(s) in Figure 2 has less surface area than the Mg(s) in Figure 1
▶️Answer/Explanation
Ans:C
Question
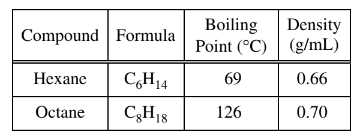
A student obtains a mixture of the liquids hexane and octane, which are miscible in all proportions. Which of the following techniques would be best for separating the two components of the mixture, and why?
(A) Filtration, because the different densities of the liquids would allow one to pass through the filter paper while the other would not.
(B) Paper chromatography, because the liquids would move along the stationary phase at different rates owing to the difference in polarity of their molecules.
(C) Column chromatography, because the higher molar mass of octane would cause it to move down the column faster than hexane.
(D) Distillation, because the liquids would boil at different temperatures owing to the difference in strength of their intermolecular forces.
▶️Answer/Explanation
Ans:D
Question
The phenomenon used to differentiate colloids and true solutions is called the __________ effect.
A) van’t Hoff B) Osmotic C) Raoult D) Henry E) Tyndall
▶️Answer/Explanation
Ans: E