Question
\(CaO(s)+H_{2}O(l)\rightarrow Ca(OH)_{2}(s) \Delta H^{\circ}_{rxn}=-63.7kj/mol_{rxn}\)
Calcium oxide, CaO(s), has been proposed as a substance that can be used to heat water quickly for portable heating packs or for cooking. When placed in water, CaO(s) reacts as shown by the equation above.
(a) A student wants to design a heating pad that could heat a 150.0 g sample of water from \(25.0^{\circ}C to 60.0^{\circ}C. 4.18J/(g.^{\circ}C).) \)
(i) Calculate the amount of heat, in joules, that the water must absorb for its temperature to change by this amount. (Assume that the specific heat capacity of the water is \(4.18 J/(g.^{\circ}C)\)
(ii) Calculate the minimum mass of CaO(s) that the student would need to use in order to cause this temperature change.
(b) The student hypothesizes that the design of the heating pad could be changed to enable it to heat 150.0 g of water from \(25.0^{\circ}C to 90.0^{\circ}C\) by using a greater mass of CaO(s).
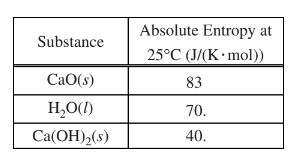
(i) Use the data in the table below to determine the standard entropy change, \(\Delta S^{\circ}_{rxn}, in J/(K.mol_{rxn}) \Delta S^{\circ}_{rxn} and \Delta H^{\circ}_{rxn} \)for the reaction.
(ii) Is the reaction thermodynamically favorable at 90.0°C? Justify your answer with a calculation. (Assume that both \(\Delta S^{\circ}_{rxn}\) and \( \Delta H^{\circ}_{rxn}\) are constant between 25.0°C and 90.0°C.) The student learns that the \(Ca(OH)_2\) produced from the reaction is relatively insoluble and that it dissolves in water according to the equation below.
\(Ca(OH)_{2}(s)\rightarrow Ca^{2+}(aq)+2OH^{-}(aq)\)
(c) The student prepares a saturated solution of Ca(OH)2 and determines that the \([Ca^{2+}] \)is 0.011 M. Calculate
the value of\( K_{sp}\) for \(Ca(OH)_{2}\)
The student wishes to significantly increase the molar solubility of the. \(Ca(OH)_{2}(s)\) and has access to the following substances.
15 mL of distilled water 15 mL of 1.0 M KOH(aq)
15 mL of 1.0 M\( CaCl_2\)(aq) 15 mL of 1.0 M HCl(aq)
(d) Which substance, when added to the \(Ca(OH)_2\) mixture, would increase the molar solubility most significantly? Justify your choice based on concepts of chemical equilibrium, such as Le Châtelier’s principle.
▶️Answer/Explanation
a(i)\(q=mc\Delta T = 150.0 g x 4.18 J/(g °C) x (60.0°C -25.0°C) = 2.19 x 104 J\)
a(ii)
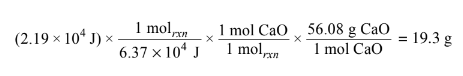
b(i) \(\Delta S°_rxn \) = ∑S° products – ∑S° reactants
= \(40. J/(K.mol) – [83 J/(K.mol) + 70. J/(K.mol)]\)
= \(-113 J/(K.mol_{rxn})\)
b(ii) Yes, because \(\Delta G° is negative. \Delta G° = \Delta H° – T \Delta S° =-63.7 kJ/mol_{rxn} K (-113 J/(K.mol_{rxn})) ×\frac{1kj}{1000j} = -22.7 kJ/mol_{rxn}\)
(c) \( [OH^-] = 2 × [Ca^2+] = 2 (0.011 M) = 0.022 \)\(M K_sp=[Ca^2+] [OH^-]^2 = (0.011)(0.022)^2 = 5.3 × 10^-6\)
(d) HCl
The \(H^+\) from the HCl reacts with \(OH^−\), decreasing \([OH^−]\). The loss of\( OH^−\) results in non-equilibrium conditions in the\( Ca(OH)_2\) dissolution. More\( Ca(OH)_2\) must dissolve to increase\( [OH^{−}]\), increasing the molar solubility as Q approaches K and the system returns to equilibrium.
Question
\(3Ag(s)+4HNO_{3}(aq)\rightarrow 3AgNO_{3}(aq)+NO(g)+2H_{2}O(l)\)
A student investigates the reaction between Ag(s) and \(HNO_3\)(aq) represented by the equation above.
(a) Predict the sign of the entropy change, ΔS°, for the reaction. Justify your answer.
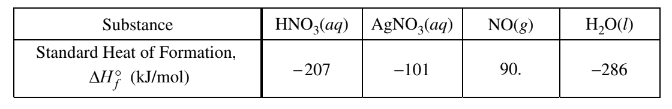
(b) Use the information in the table below to calculate the value of \(\Delta H^{\circ}_{rxn}\), the standard enthalpy change for the reaction, in\( kJ/mol_{rxn}\) .
(c) Based on your answers to parts (a) and (b), is the reaction more likely to be thermodynamically favorable at 25°C, or at 95°C? Justify your answer.
(d) The student runs the reaction using a 3 to 4 mole ratio of Ag(s) to\( HNO_3\)(aq). Suggest a method the student can use to isolate solid \(AgNO_3 \)from the other products of the reaction.
▶️Answer/Explanation
(a) The entropy change is positive because the reaction has one mole of gas in the products and none in the reactants.
(b)\( \Delta H^{\circ}_{rxn} = 3(-101) + 90. +2(-286) -4(-207)
= 43 kJ/molrxn\)
(c) \(\Delta G^{\circ}=\Delta H^{\circ}-T\Delta S^{\circ}\) The reaction is more likely to be favorable at \(95G^{\circ}\). At the higher temperature, the term\( T\Delta S^{\circ}\) is larger and positive; thus, when subtracted from \(\Delta H^{\circ}\), the value of \(\Delta G^{\circ} \) is more likely to be negative.
(d) The student can evaporate the water, leaving behind solid silver nitrate.
Question
\(3Ag(s)+4HNO_{3}(aq)\rightarrow 3AgNO_{3}(aq)+NO(g)+2H_{2}O(l)\)
A student investigates the reaction between Ag(s) and \(HNO_3\)(aq) represented by the equation above.
(a) Predict the sign of the entropy change, ΔS°, for the reaction. Justify your answer.
(b) Use the information in the table below to calculate the value of \(\Delta H^{\circ}_{rxn}\), the standard enthalpy change for the reaction, in\( kJ/mol_{rxn}\) .

(c) Based on your answers to parts (a) and (b), is the reaction more likely to be thermodynamically favorable at 25°C, or at 95°C? Justify your answer.
(d) The student runs the reaction using a 3 to 4 mole ratio of Ag(s) to\( HNO_3\)(aq). Suggest a method the student can use to isolate solid \(AgNO_3 \)from the other products of the reaction.
▶️Answer/Explanation
(a) The entropy change is positive because the reaction has one mole of gas in the products and none in the reactants.
(b)\( \Delta H^{\circ}_{rxn} = 3(-101) + 90. +2(-286) -4(-207)
= 43 kJ/molrxn\)
(c) \(\Delta G^{\circ}=\Delta H^{\circ}-T\Delta S^{\circ}\) The reaction is more likely to be favorable at \(95G^{\circ}\). At the higher temperature, the term\( T\Delta S^{\circ}\) is larger and positive; thus, when subtracted from \(\Delta H^{\circ}\), the value of \(\Delta G^{\circ} \) is more likely to be negative.
(d) The student can evaporate the water, leaving behind solid silver nitrate.
