Question
Reaction 1: \(FeO(s)+CO(g)→Fe(l)+CO_2(g)\) ΔG°\(_{rxn}>0\)
Reaction 2: \(C(s)+CO_2(g)→2CO(g)\) ΔG°\(_{rxn}<0\)
Overall reaction: \(FeO(s)+C(s)→Fe(l)+CO(g)\) ΔG°\(_{rxn}<0\)
The chemical equations above represent the main reactions that occur during the production of Fe(l) under certain conditions. The overall reaction couples reactions 1 and 2, resulting in a thermodynamically favorable process. Which of the following best explains whether or not a particle diagram could represent how the coupling of reaction 1 and reaction 2 results in ΔG°\(_{rxn}<0\) ?
A A particle diagram that represents the increase in the volume of gaseous product particles would be a good representation of how the coupling of reactions 1 and 2 results in a thermodynamically favorable process.
B A particle diagram that represents the decrease in the average kinetic energy of the particles would be a good representation of how the coupling of reactions 1 and 2 results in a thermodynamically favorable process.
C A particle diagram cannot represent how the changes in energy that take place as reaction 1 occurs are more than offset by the changes in energy taking place as reaction 2 occurs, resulting in a thermodynamically favorable overall reaction.
D A particle diagram cannot represent the changes in the amount of matter that take place as reaction 1 is coupled to reaction 2, resulting in a thermodynamically favorable overall reaction.
▶️Answer/Explanation
Ans:C
ΔG° represents the energy that can be obtained from a reaction to do work. Particle diagrams cannot represent changes in energy and are best used to represent changes in the relative arrangements of atoms in substances (changes in matter).
Question
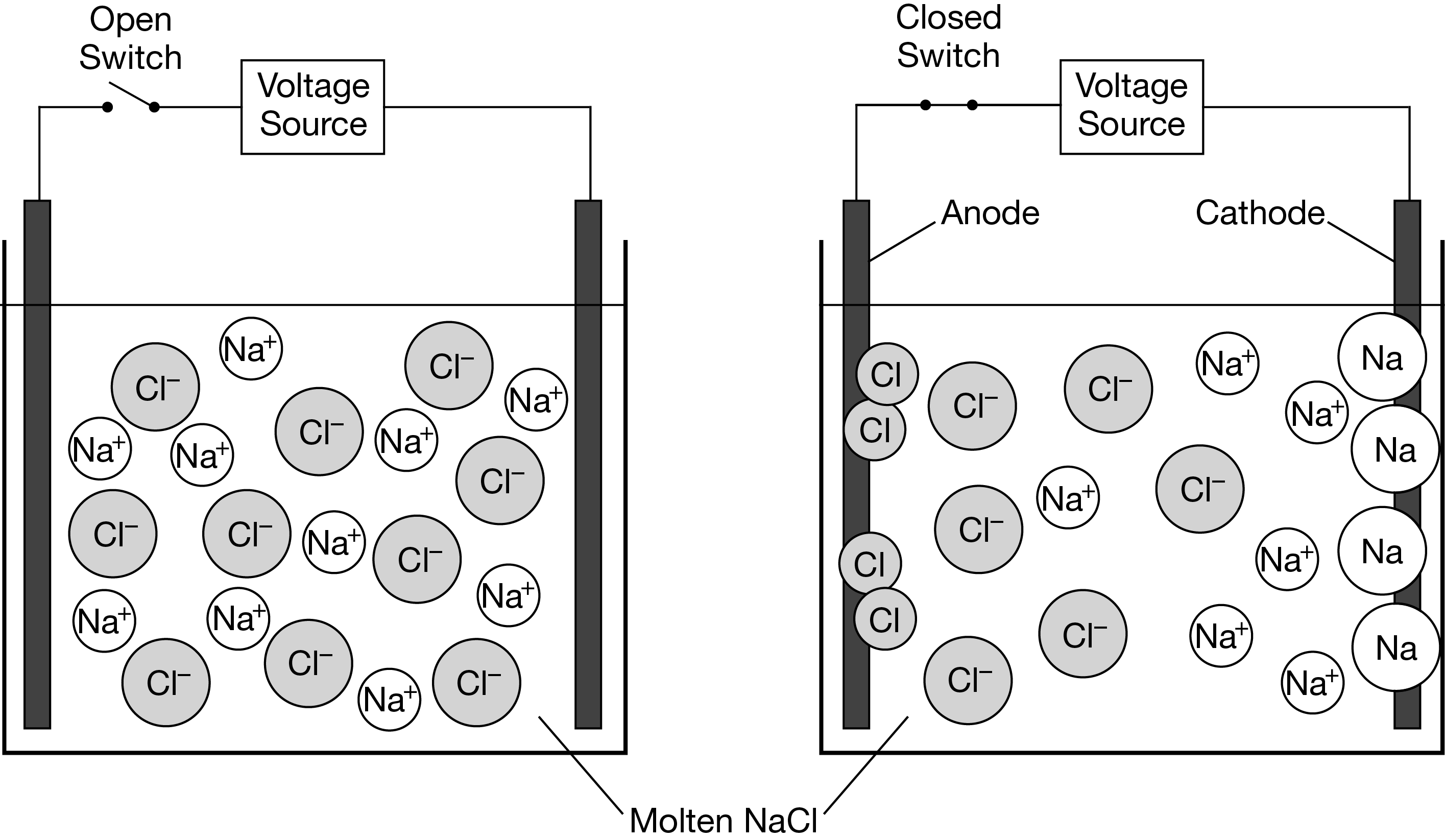
The particle diagrams above represent \(NaCl(l)\) and its decomposition into \(Na(l)\) and \(Cl_2(g)\) in an electrochemical cell after voltage is applied. Which of the following statements about the thermodynamic favorability of the decomposition of \(NaCl(l)\) is supported by the particle diagrams and why?
A The decomposition is thermodynamically favored because the transfer of electrons to and from the ions occurs at the electrodes.
B The decomposition is thermodynamically favored because the formation of a gaseous product results in an increase in entropy.
C The decomposition is not thermodynamically favored because the pure elements form only after electrical energy is supplied.
D The decomposition is not thermodynamically favored because there is a decrease in the number of particles as the reaction proceeds.
▶️Answer/Explanation
Ans:C
Even at the high temperatures required to melt \(NaCl(s)\) , the diagrams show that the decomposition is not thermodynamically favored and does not occur unless electrical energy is supplied.
Question
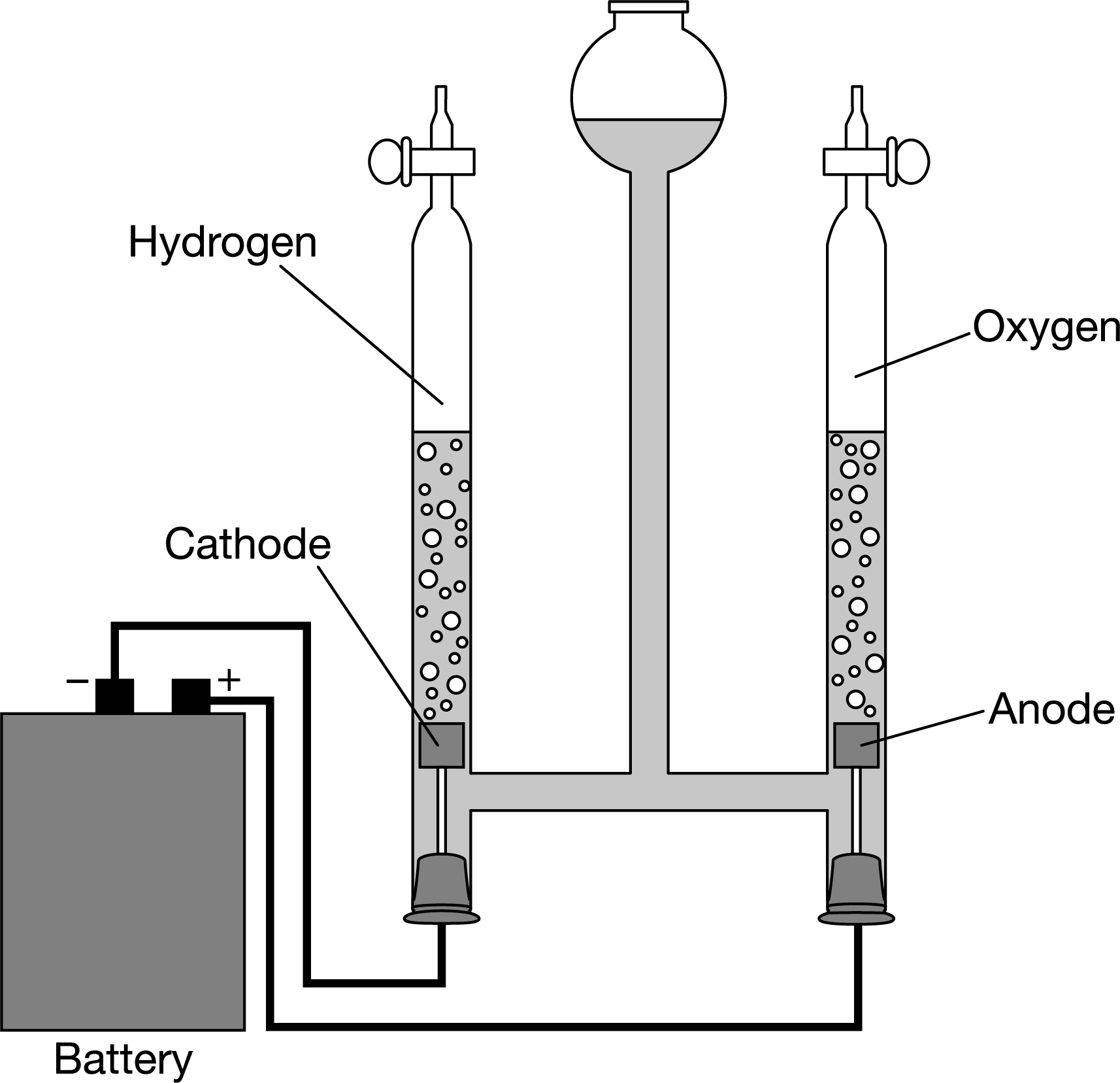
\( 2H_2O(l)→2H_2(g)+O_2(g)\)
The reaction in which \(H_2O(l)\) is decomposed into \(H_2(g)\) and \(O_2(g)\) is thermodynamically unfavorable (ΔG°>0 ). However, an electrolytic cell, such as the one represented above, can be used to make the reaction occur. Which of the following identifies a flaw in the representation?
A Oxidation is occurring at the anode.
B The equation is not correctly balanced.
C Electrical energy is needed for the reaction to proceed.
D The volumes of collected gas in each tube should not be the same.
▶️Answer/Explanation
Ans:D
According to the stoichiometry of the electrolysis reaction, twice as many hydrogen molecules are produced as oxygen molecules. Because the volume of two gases under the same conditions is proportional to the number of gas particles present, the volume of the \(H_2(g)\) collected in the cell diagram should be twice that of the \(O_2(g)\).
