Question
A student is studying the properties of CaSO4 and PbSO4. The student has samples of both compounds, which are white powders.
(a) The student tests the electrical conductivity of each solid and observes that neither solid conducts electricity. Describe the structures of the solids that account for their inability to conduct electricity.
The student places excess CaSO4 (s) in a beaker containing 100 mL of water and places excess PbSO4 (s) in another beaker containing 100 mL of water. The student stirs the contents of the beakers and then measures the electrical conductivity of the solution in each beaker. The student observes that the conductivity of the solution in the beaker containing the CaSO4 (s) is higher than the conductivity of the solution in the beaker containing the PbSO4 (s).
(b) Which compound is more soluble in water, CaSO4(s) or PbSO4(s) ? Justify your answer based on the results of the conductivity test.
The left side of the diagram below shows a particulate representation of the contents of the beaker containing the CaSO4(s) from the solution conductivity experiment.
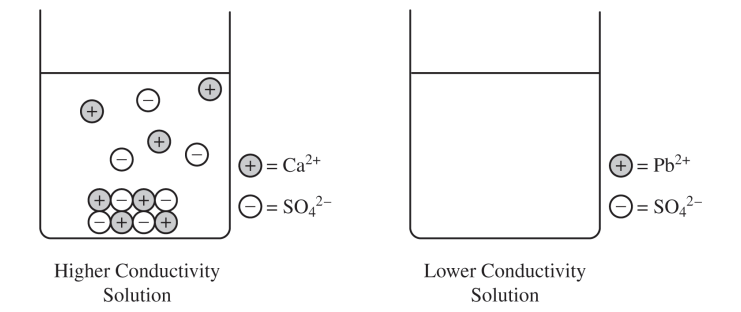
(c) Draw a particulate representation of PbSO4(s) and the ions dissolved in the solution in the beaker on the right in the diagram. Draw the particles to look like those shown to the right of the beaker. Draw an appropriate number of dissolved ions relative to the number of dissolved ions in the beaker on the left.
(d) The student attempts to increase the solubility of CaSO4(s) by adding 10.0 mL of 2 M H2SO4 (aq) to the beaker, and observes that additional precipitate forms in the beaker. Explain this observation.
▶️Answer/Explanation
Ans:
(a) For a correct description:
Ionic solids do not have free-moving ions that are required to carry an electric current. Therefore, there is no conduction of electricity.
(b) For the correct answer and a valid justification:
CaSO4. The greater electrical conductivity of the CaSO4 solution relative to the PbSO4 solution implies a higher concentration of ions, which comes from the dissolution (dissociation) of CaSO4 to a greater extent.
(c) For a correct drawing that shows an equal number of cations and anions:
The drawing shows solid PbSO4 at the bottom of the beaker (similar to the solid shown for CaSO4) and fewer dissociated Pb2+ and SO42− ions in the solution.
(d) For a correct explanation:
The additional precipitate is CaSO4 that forms in response to the increased [SO42−] in solution. According to Le Chatelier’s principle (Q > Ksp), the introduction of SO42− as a
common ion shifts the equilibrium towards the formation of more CaSO4(s).
Question
A student is studying the properties of CaSO4 and PbSO4. The student has samples of both compounds, which are white powders.
(a) The student tests the electrical conductivity of each solid and observes that neither solid conducts electricity. Describe the structures of the solids that account for their inability to conduct electricity.
The student places excess CaSO4 (s) in a beaker containing 100 mL of water and places excess PbSO4 (s) in another beaker containing 100 mL of water. The student stirs the contents of the beakers and then measures the electrical conductivity of the solution in each beaker. The student observes that the conductivity of the solution in the beaker containing the CaSO4 (s) is higher than the conductivity of the solution in the beaker containing the PbSO4 (s).
(b) Which compound is more soluble in water, CaSO4(s) or PbSO4(s) ? Justify your answer based on the results of the conductivity test.
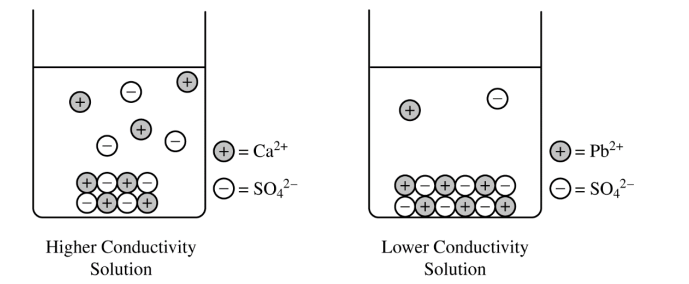
The left side of the diagram below shows a particulate representation of the contents of the beaker containing the CaSO4(s) from the solution conductivity experiment.

(c) Draw a particulate representation of PbSO4(s) and the ions dissolved in the solution in the beaker on the right in the diagram. Draw the particles to look like those shown to the right of the beaker. Draw an appropriate number of dissolved ions relative to the number of dissolved ions in the beaker on the left.
(d) The student attempts to increase the solubility of CaSO4(s) by adding 10.0 mL of 2 M H2SO4 (aq) to the beaker, and observes that additional precipitate forms in the beaker. Explain this observation.
▶️Answer/Explanation
Ans:
(a) For a correct description:
Ionic solids do not have free-moving ions that are required to carry an electric current. Therefore, there is no conduction of electricity.
(b) For the correct answer and a valid justification:
CaSO4. The greater electrical conductivity of the CaSO4 solution relative to the PbSO4 solution implies a higher concentration of ions, which comes from the dissolution (dissociation) of CaSO4 to a greater extent.
(c) For a correct drawing that shows an equal number of cations and anions:
The drawing shows solid PbSO4 at the bottom of the beaker (similar to the solid shown for CaSO4) and fewer dissociated Pb2+ and SO42− ions in the solution.

(d) For a correct explanation:
The additional precipitate is CaSO4 that forms in response to the increased [SO42−] in solution. According to Le Chatelier’s principle (Q > Ksp), the introduction of SO42− as a
common ion shifts the equilibrium towards the formation of more CaSO4(s).
Question
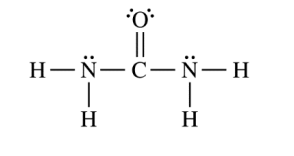
The compound urea, H2NCONH2 , is widely used in chemical fertilizers. The complete Lewis electron-dot diagram for the urea molecule is shown above.
(a) Identify the hybridization of the valence orbitals of the carbon atom in the urea molecule.
(b) Urea has a high solubility in water, due in part to its ability to form hydrogen bonds. A urea molecule and four water molecules are represented in the box below. Draw ONE dashed line (—-) to indicate a possible location of a hydrogen bond between a water molecule and the urea molecule.

The dissolution of urea is represented by the equation above. A student determines that 5.39 grams of H2NCONH2 (molar mass 60.06 g/mol) can dissolve in water to make 5.00 mL of a saturated solution at 20.°C.
(c) Calculate the concentration of urea, in mol/L, in the saturated solution at 20.°C.
(d) The student also determines that the concentration of urea in a saturated solution at 25°C is 19.8 M. Based on this information, is the dissolution of urea endothermic or exothermic? Justify your answer in terms of Le Chatelier’s principle.
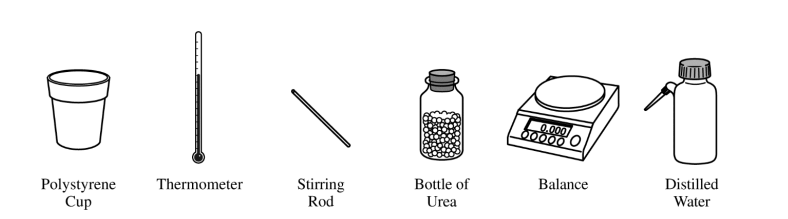
(e) The equipment shown above is provided so that the student can determine the value of the molar heat of solution for urea. Knowing that the specific heat of the solution is 4.18 J/(g⋅°C), list the specific measurements that are required to be made during the experiment.
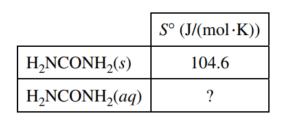
(f) The entropy change for the dissolution of urea, ΔS0soln , is 70.1 J/(mol⋅K) at 25°C. Using the information in the table above, calculate the absolute molar entropy, S°, of aqueous urea.
(g) Using particle-level reasoning, explain why ΔS0soln is positive for the dissolution of urea in water.
(h) The student claims that ΔS° for the process contributes to the thermodynamic favorability of the dissolution of urea at 25°C. Use the thermodynamic information above to support the student’s claim.
▶️Answer/Explanation
Ans:
(a)
| sp2 |
(b)
| A dashed line should connect a hydrogen atom in water to a nitrogen or oxygen atom in urea or an oxygen atom in water to a hydrogen atom in urea. One possible correct response is shown above. |
(c)
5.39 g H2NCONH2 × \(\frac{1 mol}{60.06 g}=0.0897 mol\) \(\frac{0.0897 mol}{0.00500 L}=17.9 M\) |
(d)
| The increased solubility at the higher temperature implies that the dissolution of urea is endothermic. If a saturated solution of urea is heated, then the equilibrium system is stressed. The stress is counteracted by the endothermic dissolution of more urea. |
(e)
| mass of urea, mass of water, initial temperature of water, final temperature of solution |
(f)
ΔS0soln = S0 (H2NCONH2(aq)) – S0 (H2NCONH2(s)) 70.1 J/(mol.K) = S0 (H2NCONH2(aq)) – 104.6 J/(mol.K) S0 (H2NCONH2(aq)) = 174.7 J/(mol.K) |
(g)
| Urea molecules in solution have a greater number of possible arrangements than in solid urea. This increased number of arrangements corresponds to a positive ΔS0soln. |
(h)
| Thermodynamic favorability for a process at standard conditions is determined by the sign of ΔG0 , with ΔG0 =ΔH0 -TΔS0 . Since ΔS0 is positive, the TΔS0 term makes the value of ΔG0 smaller and thus makes the dissolution more thermodynamically favorable. |
