Question
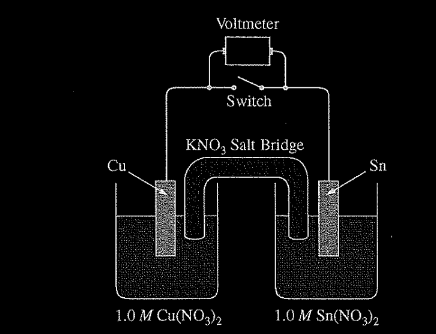
A student is given a standard galvanic cell, represented above, that has a Cu electrode and a Sn electrode. As current flows through the cell, the student determines that the Cu electrode increases in mass and the Sn electrode decreases in mass.
(a) Identify the electrode at which oxidation is occurring. Explain your reasoning based on the student’s observations.
(b) As the mass of the Sn electrode decreases, where does the mass go?
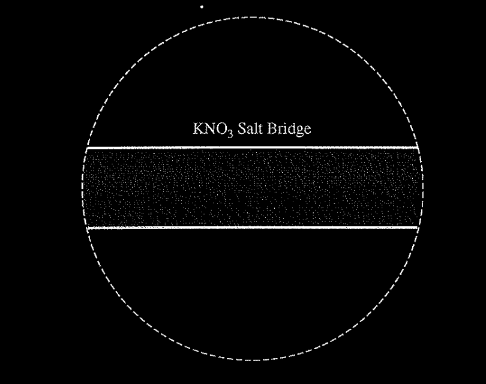
(c) In the expanded view of the center portion of the salt bridge shown in the diagram below, draw and label a particle view of what occurs in the salt bridge as the cell begins to operate. Omit solvent molecules and use arrows to show the movement of particles.
d) A nonstandard cell is made by replacing the 1.0 M solutions of Cu\((NO_3)_2\) and Sn\((NO_3)_2\) in the standard cell with 0.50 M solutions of Cu(NO3)2 and Sn\((NO_{3})_{2}\). The volumes of solutions in the nonstandard cell are identical to those in the standard cell.
(i) Is the cell potential of the nonstandard cell greater than, less than, or equal to the cell potential of the standard cell? Justify your answer.
(ii) Both the standard and nonstandard cells can be used to power an electronic device. Would the nonstandard cell power the device for the same time, a longer time, or a shorter time as compared with the standard cell? Justify your answer.
(e) In another experiment, the student places a new Sn electrode into a fresh solution of 1.0 M\( Cu(NO_{3})_{2}\) ·
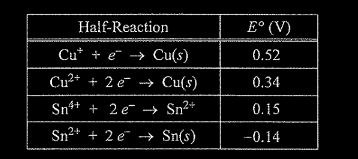
(i) Using information from the table above, write a net-ionic equation for the reaction between the Sn electrode and the Cu\((NO_3)_2\) solution that would be thermodynamically favorable. Justify that the reaction is thermodynamically favorable.
(ii) Calculate the value of \(\Delta G\) for the reaction. Include units with your answer.
▶️Answer/Explanation
(a) Since the Sn electrode is losing mass, Sn atoms must be forming \(Sn^{2+}\)(aq). This process is oxidation. OR because the cell operates, E° must be positive and, based on the E° values from the table, it must be Sn that is oxidized.
(b) The atoms on the Sn electrode are going into the solution as \(Sn^{2+}\) ions.
(c) The response should show at least one \(K^+\) ion moving toward the Cu compartment on the left and at least one \(NO_{3}^-\) ion moving in the opposite direction.
d(i) It is the same. In the cell reaction \(Q= \frac{[Sn^{2+}]}{ [Cu^{2+}]}\) , and the concentrations of \(Sn^{2+}\) and \(Cu^{2+}\) are equal to each other in both cases.
d(ii) The nonstandard cell would power the device for a shorter time because the supply of \(Cu^{2+}\) ions will be exhausted more quickly. OR The nonstandard cell would power the device for a shorter time because the reaction will reach E=0 more quickly.
e (i) \(Cu^{2+}(aq) + Sn(s) → Cu(s) + Sn^{2+}\)(aq) E° is positive \((0.34 V + 0.14 V = 0.48 V)\), therefore the reaction is thermodynamically favorable. OR The cell observations from earlier parts of the question are evidence that the Sn is oxidized and Cu is reduced, therefore E° must be positive.
e.(ii) \(\Delta G^0=-nFE^0\)
\(\Delta G^0=-\frac{2 mol e^-}{mol_{rxn}}\times \frac{96,485}{mol e^-}\times \frac{0.48}{c}=-93,000 J/mol_{rxn}\)
Question
Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory.
(a) Consider the reaction represented below, which is spontaneous at 298 K.
\(CO_2(g) + 2NH_3(g) \rightarrow CO(NH_2)_2(s) + H_2O(l)\) \(\Delta H_{298}^o = -134\) kj
(i) For the reaction, indicate whether the standard entropy change, \(\Delta S_{298}^o\), is positive, or negative, or zero, Justify your answer.
(ii) Which factor, the change in enthalpy, \(\Delta H_{298}^o\), or the change in entropy, \(\Delta S_{298}^o\), provides the principal driving force for the reaction at 298 K? Explain.
(iii) For the reaction, how is the value of the standard free energy change, \(\Delta G^o\), affected by an increase in temperature? Explain.
(b) Some reactions that are predicted by their sign of \(\Delta G^o\) to be spontaneous at room temperature do not proceed at a measurable rate at room temperature.
(i) Account for this apparent contradiction.
(ii) A suitable catalyst increases the rate of such a reaction. What effect does the catalyst have on \(\Delta G^o\) for the reaction ? Explain.
▶️Answer/Explanation
Answer:
(a) (i) \(\Delta S^o\) is negative (-) OR \(\Delta S^o\) < 0 OR entropy is decreasing
3 moles of gaseous particles are converted to 2 moles of solid/liquid.
- One point earnedfor correct identi&ation of (-) sign of \(\Delta S^o\)
- One point earnedfor correct explanation (mention ofphases is crucial for point)
- No point earned if incorrect \(\Delta S^o\) sign is obtainedfiom the presumed value of \(\Delta G^o\)
(ii) \(\Delta H^o\) drives the reaction.
The decrease in entropy \((\Delta S^o < 0)\) cannot drive the reaction, so the decrease in enthalpy \((\Delta H^o < 0)\) MUST drive the reaction.
OR
\(\Delta G^o = \Delta H^o – T \Delta S^o\); for a spontaneous reaction \(\Delta G^o < 0\), and negative value of \(\Delta S^o\) causes a positive \(\Delta G^o\).
- One point earnedfor identtfiing \(\Delta H^o\) as the principal driving force for the reaction
- One point earnedfor correct justification
- Justification point earned by mentioning the eflects of changes in entropy and enthalpy on the
spontaneity of the reaction OR by a mathematical argument using the Gibbs-Helmholtz equation
and some implication about the comparison between the effects of \(\Delta S^o\) and \(\Delta H^o\)
(iii) Given that \(\Delta G^o = T \Delta S^o\) and \(\Delta S^o < 0\), an increase in temperature causes an increase in the value of \(\Delta G^o\) (\(\Delta G^o\) becomes less negative).
One point earnedfor the description of the effect of an increase in temperature on \(\Delta S^o\) and
consequently on \(\Delta G^o\)
No point earnedfor an argument based on Le Chatelier s principle
(b) (i) The reaction rate depends on the reaction kinetics, which is determined by the value of the
activation energy, \(E_{act}\). If the activation energy is large, a reaction that is thermodynamically
spontaneous may proceed very slowly (if at all).
- One point earnedfor linking the rate of the reaction to the activation energy, which may be
explained verbally or shown on a reaction proJle diagram
(ii) The catalyst has no effect on the value of \(\Delta G^o\).
The catalyst reduces the value of \(E_{act}\), increasing the rate of reaction, but has no effect on the
values of \(\Delta H^o\) and \(\Delta S^o\), so it cannot affect the thermodynamics of the reaction.
- One point earnedfor indicating no change in the value of \(\Delta G^o\)
- One point earnedfor indicating (verbally, or with a reaction-procfile diagram) that the
catalyst affects the activation energy
