Question
HCOOH(aq ) + H2O(l) ⇔ H2O+(aq) + HCOO– (aq) Ka = 1.8 × 10-4
Methanoic acid, HCOOH, ionizes according to the equation above.
(a) Write the expression for the equilibrium constant, Ka, for the reaction.
(b) Calculate the pH of a 0.25 M solution of HCOOH.
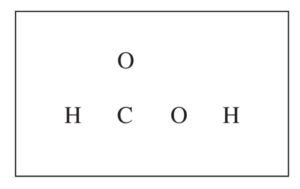
(c) In the box below, complete the Lewis electron-dot diagram for HCOOH. Show all bonding and nonbonding valence electrons.
H2NNH2(aq) + H2O(l) ⇔ H2NNH3+ (aq) + OH –(aq) Kb = 1.3 × 10-6
(d) In aqueous solution, the compound H2NNH2 reacts according to the equation above. A 50.0 mL sample of 0.25 M H2NNH2(aq) is combined with a 50.0 mL sample of 0.25 M HCOOH(aq).
(i) Write the balanced net ionic equation for the reaction that occurs when H2NNH2 is combined with HCOOH.
(ii) Is the resulting solution acidic, basic, or neutral? Justify your answer.
When a catalyst is added to a solution of HCOOH(aq), the reaction represented by the following equation occurs.
HCOOH(aq) → H2 (g) + CO2 (g)
(e) Is the reaction a redox reaction? Justify your answer.
(f) The reaction occurs inarigid 4.3 L vessel at 25°C, and the total pressure is monitored, as shown in the graph above. The vessel originally did not contain any gas. Calculate the number of moles of CO2(g) produced in the reaction. (Assume that the amount of CO2(g) dissolved in the solution is negligible.)
(g) After the reaction has proceeded for several minutes, does the amount of catalyst increase, decrease, or remain the same? Justify your answer.
▶️Answer/Explanation
Ans:
(a)
For the correct expression:
\(K_{a}=\frac{[H_{3}O^{+}][HCOO^{-}]}{[HCOOH]}\)
(b) For the correct calculated concentration of H3O+ :
HCOOH + H2O ⇔ H3O+ + HCOO–
I 0.25 0 0
C –x +x +x
E 0.25 – x x x
Let [H3 O+ ] = x, then 1.8 × 10-4 \(\frac{x^{2}}{(0.25-x)}\)
Assume x << 0.25, then 1.8 × 10-4 \(\frac{x^{2}}{(0.25)}\) ⇒ x = 0.0067 M
For the correct calculated value of pH:
pH = -log[H3 O + ] = – log(0.0067) = 2.17
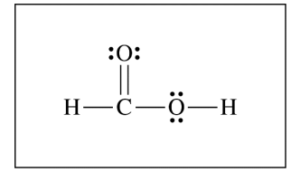
(c) For the correct diagram:
(d) (i) For the correct balanced equation (state symbols not required):
H2NNH2 (aq) + HCOOH(aq) → H2NNH3+ (aq) + HCOO– (aq)
(ii) For the correct answer and a valid justification:
Acidic. The Ka of H2NNH3+ is greater than the Kb of HCOO−, so the production of H3O+(aq) occurs to a greater extent than the production of OH−(aq).
(e) For the correct answer and a valid justification:
Accept one of the following:
• Yes. The oxidation number of hydrogen changes from +1 in HCOOH to zero in H2.
• Yes. The oxidation number of carbon changes from +2 in HCOOH to +4 in CO2.
(f) For the correct calculated value of the pressure of CO2 (may be implicit):
24 atm total × 1 atm CO2 / 2 atm of product = 12 atm CO2
For the correct calculated number of moles of CO2:
PV = nRT
\(n = \frac{PV}{RT}=\frac{(12 atm)(4.3 L)}{(0.08206 L atm mol^{-1}K^{-})(298 K)}=2.1 mol CO_{2}\)
(g) For the correct answer and a valid justification:
It would remain the same. In a catalyzed reaction the net amount of catalyst is constant.
Question
NaHCO3(s) + HC2H3O2(aq) → NaC2H3O2(aq) + H2O(l) + CO2(g)
A student designs an experiment to study the reaction between NaHCO3 and HC2H3O2 . The reaction is represented by the equation above. The student places 2.24 g of NaHCO3 in a flask and adds 60.0 mL of 0.875 M HC2H3O2 . The student observes the formation of bubbles and that the flask gets cooler as the reaction proceeds.
(a) Identify the reaction represented above as an acid-base reaction, precipitation reaction, or redox reaction. Justify your answer.
(b) Based on the information above, identify the limiting reactant. Justify your answer with calculations.
(c) The student observes that the bubbling is rapid at the beginning of the reaction and gradually slows as the reaction continues. Explain this change in the reaction rate in terms of the collisions between reactant particles.
(d) In thermodynamic terms, a reaction can be driven by enthalpy, entropy, or both.
(i) Considering that the flask gets cooler as the reaction proceeds, what drives the chemical reaction between NaHCO3(s) and HC2H3O2(aq) ? Answer by drawing a circle around one of the choices below.
Enthalpy only Entropy only Both enthalpy and entropy
(ii) Justify your selection in part (d)(i) in terms of ΔG°.
(e) The HCO3− ion has three carbon-to-oxygen bonds. Two of the carbon-to-oxygen bonds have the same length and the third carbon-to-oxygen bond is longer than the other two. The hydrogen atom is bonded to one of the oxygen atoms. In the box below, draw a Lewis electron-dot diagram (or diagrams) for the HCO3− ion that is (are) consistent with the given information.

(f) A student prepares a solution containing equimolar amounts of HC2H3O2 and NaC2H3O2 . The pH of the solution is measured to be 4.7. The student adds two drops of 3.0 M HNO3(aq) and stirs the sample, observing that the pH remains at 4.7. Write a balanced, net-ionic equation for the reaction between HNO3(aq) and the chemical species in the sample that is responsible for the pH remaining at 4.7.
▶️Answer/Explanation
Ans:
(a)
| It is an acid-base reaction. The weak acid HC2H3O2 reacts with the weak base HCO3– with HC2H3O2 donating a proton. OR It is an acid-base reaction. No solid precipitates, so it is not a precipitation reaction. None of the oxidation numbers change, so it is not a redox reaction. |
(b)
\(2.24 g NaHCO_{3}\times \frac{1 mol NaHCO_{3}}{84.0 g}= 0.0267 mol NaHCO_{3}\) \(60.0 mL\times \frac{0.875 mol HC_{2}H_{3}O_{2}}{1000 mL}= 0.0525 mol HC_{2}H_{3}O_{2}\) The NaHCO3(s) and HC2H3O2 (aq) react in a 1:1 ratio, so the limiting reactant is NaHCO3(s). |
(c)
| As the reaction proceeds, both reactants are consumed and their concentrations decrease. Collisions between reactant particles become less likely as their concentrations decrease, thus the reaction rate slows. |
(d) (i)
| Entropy only should be circled. |
(ii)
ΔG0 = ΔH0 – TΔS0 Reactions are thermodynamically favorable when ΔG0 is negative. Since the reaction is endothermic (the flask cooler, ΔH0 is positive), the reaction is not driven by enthalpy, because enthalpy does not help make ΔG0 negative. Because there are no gases in the reactants and one of the products is a gas, ΔS0 must be positive, which helps make ΔG0 negative. |
(e)

| See diagram above. |
(f)
| C2H3O2– + H3O+ → HC2H3O2 + H2O OR C2H3O2– + H+ → HC2H3O2 |
Question
For each of the following, use appropriate chemical principles to explain the observation.
(a) Sodium chloride may be spread on an icy sidewalk in order to melt the ice; equimolar amounts of calcium chloride are even more effective.
(b) At room temperature, NH3 is a gas and H2O is a liquid, even though NH3 has a molar mass of 17 grams and H2O has a molar mass of 18 grams.
(c) C (graphite) is used as a lubricant, whereas C (diamond) is used as an abrasive.
(d) Pouring vinegar onto the white residue inside a kettle used for boiling water results in a fizzing/bubbling phenomenon.
▶️Answer/Explanation
Ans:
(a) The addition of a solute lowers the freezing point of water.
A mole of NaCl contains (dissociates into) 2 moles of ions/particles, whereas a mole of CaCl2 contains (dissociates into) 3 moles of ions. Therefore CaCl2 is more effective.
(b) Hydrogen bonding is the most important intermolecular attractive force between molecules of H2O and between molecules of NH3.
Water is a liquid because the hydrogen-bonding forces are stronger between adjacent H2O molecules than between adjacent NH3 molecules.
Further explanations for the stronger hydrogen bonding in H2O include the larger dipole moment (or more polar character) of H2O compared to NH3 and the fact that 0 is more electronegative than N is.
(c) Graphite’s structure consists of 2-dimensional sheets of covalently bonded carbon atoms. The attractive forces between sheets (layers) are weak London (dispersion) forces, which allow the sheets to slide easily over one another.
Note: must indicate layers and sliding to earn point.
Diamond consists of an extended 3-dimensional covalent network of carbon atoms. This makes diamond a very hard substance.
(d) Vinegar, a dilute solution of acteic acid, reacts with the white solid, which contains metal carbonates, in a neutralization reaction to form gaseous CO2.
