Question
Reaction 1: X⇄Y
Reaction 2: X⇄Z
X undergoes the two competing reactions represented above. The following graph shows the changes in energy that occur as the two reactions take place.
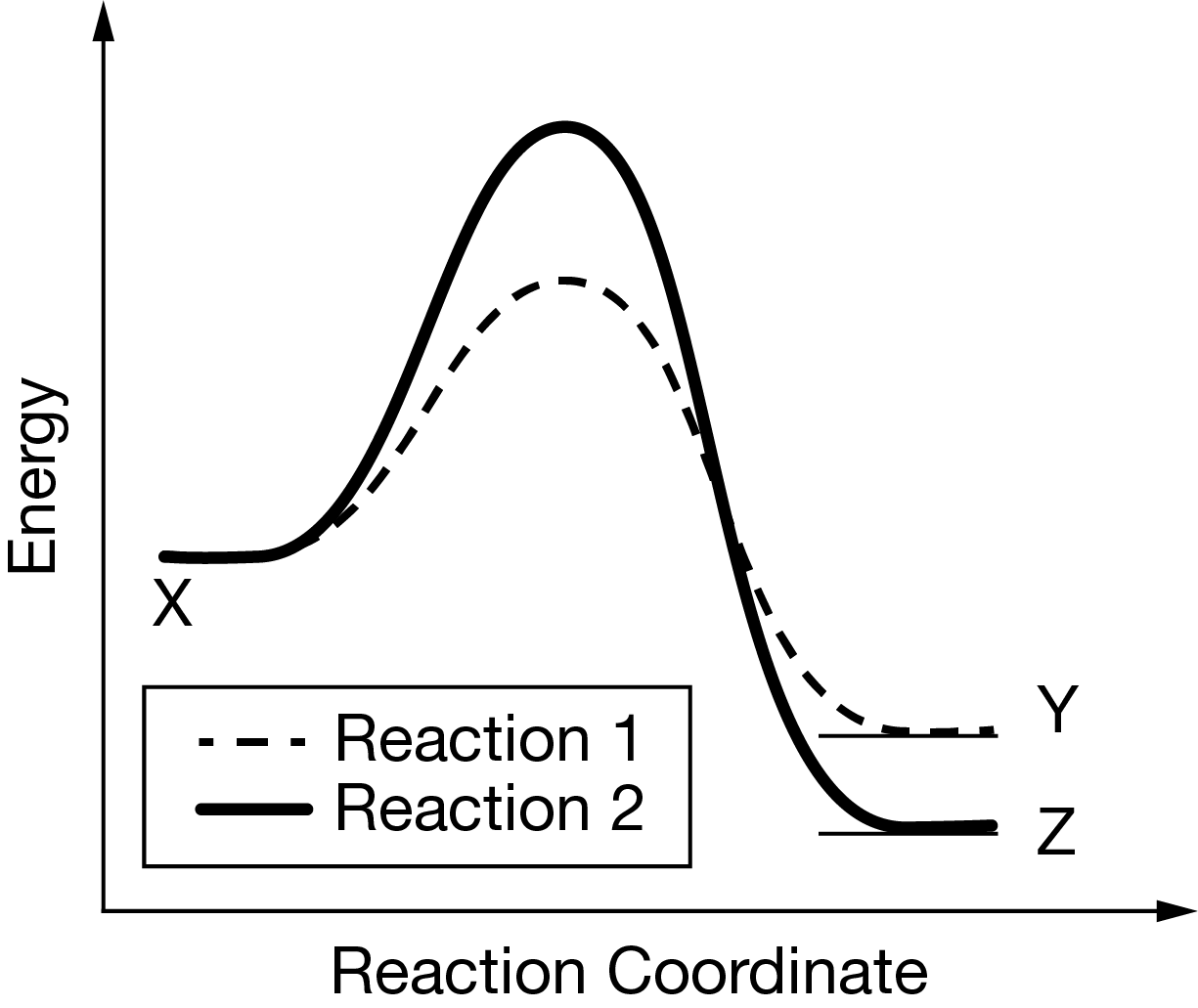
In an experiment done at a low temperature, one hour after the reaction started, Y was produced but almost no Z was produced. At a much higher temperature, one hour after the reaction started, both Y and Z had been produced. Which of the following best explains why very little Z was produced at the lower temperature?
A A smaller number of X molecules had enough energy to overcome the activation energy barrier for reaction 2, and very little Z was produced.
B The higher average kinetic energy of the X molecules at the lower temperature resulted in very little Z produced.
C The greater collision frequency between X molecules at the lower temperature resulted in very little Z produced.
D The intermolecular forces between X molecules were much weaker, and very little Z was produced.
▶️Answer/Explanation
Ans:A
Based on the graph, the activation energy of reaction 1 is much lower than the activation energy of reaction 2. At the lower temperature, the number of X molecules having enough energy to overcome the activation energy barrier was too small for reaction 2 to form Z in significant yields. Y was produced instead because a much larger number of molecules had enough energy to overcome the activation energy barrier for reaction 1.
Question
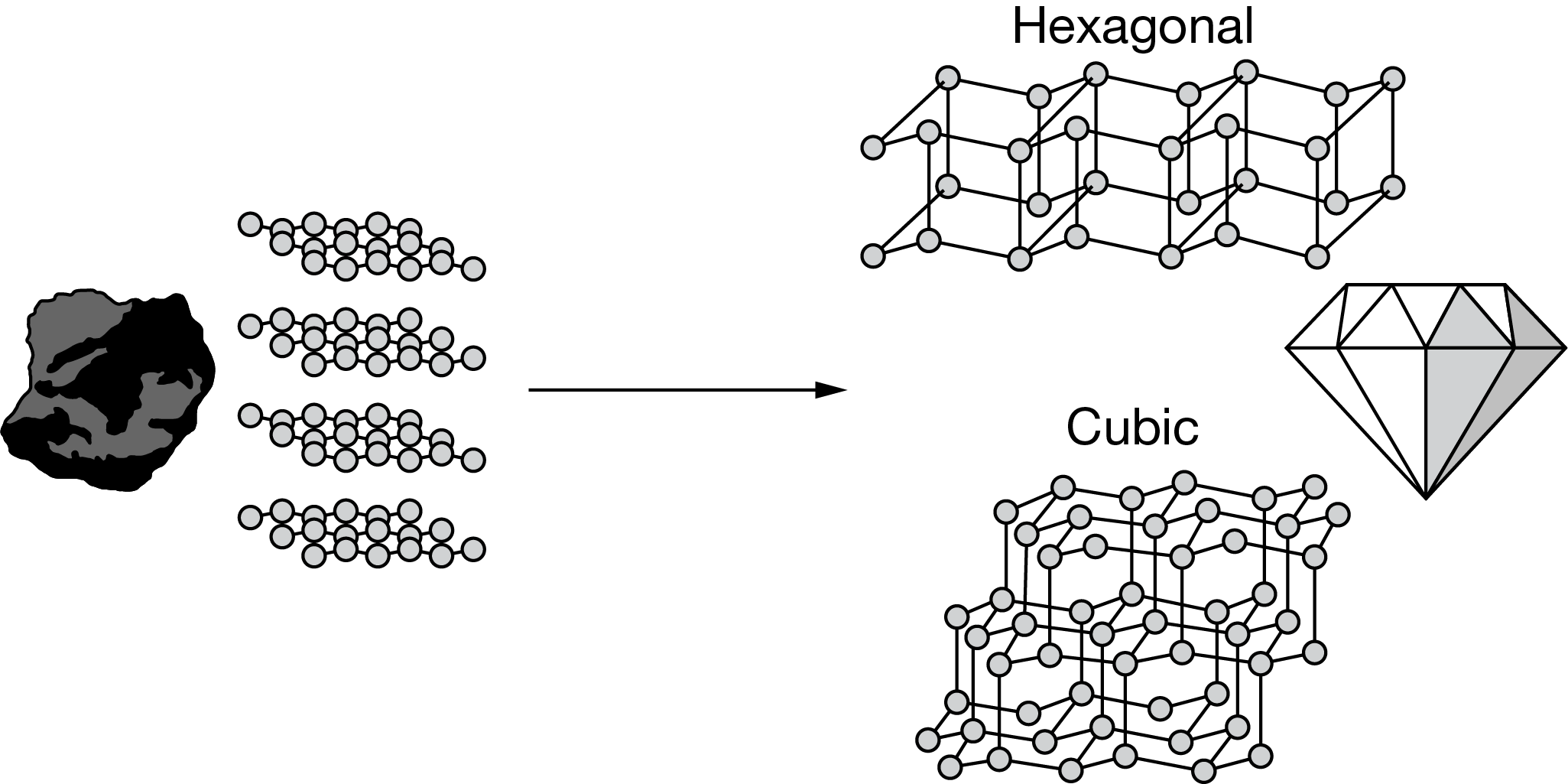
Graphite, an allotrope of carbon, is converted into cubic diamond through a process that may take a billion years or longer. As illustrated above, scientists can make synthetic diamonds using a certain process in about one week. However, these synthetic diamonds have carbon atoms in a hexagonal lattice. Diamonds with carbon atoms in a cubic lattice are not produced even though they are thermodynamically more stable than hexagonal diamond. Which of the following best justifies why the synthetic process produces hexagonal diamond and not the more thermodynamically stable cubic diamond?
A The amount of energy required to create new bonds between carbon atoms in cubic diamond is much greater than the amount of energy required to create hexagonal diamond.
B The amount of energy required to create new bonds between carbon atoms in cubic diamond is much smaller than the amount of energy required to create hexagonal diamond.
C The activation energy needed to form cubic diamond is much less than the activation energy needed to form hexagonal diamond.
D The activation energy needed to form cubic diamond is much greater than the activation energy needed to form hexagonal diamond.
▶️Answer/Explanation
Ans:D
The production of hexagonal diamond, even if it is not the most stable, indicates that factors affecting the rate of the reaction are the most important. Therefore, cubic diamond is most likely not produced because the process \(C_{graphite}→C_{cubic diamond}\)
has a much greater activation energy than \(C_{graphite}→C_{hexagonal diamond}\) and therefore an extremely slow reaction rate.
Question
\(ATP+H_2O\)⇄\(ADP+P_i\) ΔG\(=−30.5kJ/mol_{rxn}\)
The hydrolysis of adenosine triphosphate (ATP) is represented by the equation above. This reaction is critically important in cellular biology, but the reaction itself proceeds at a very slow rate. Based on the information given, which of the following best explains why an enzyme (biological catalyst) is required for the reaction to occur at a faster rate?
A Because ΔG<0 , the hydrolysis of ATP is not thermodynamically favorable. In cells, ΔS is increased by increasing the amount of H2O consumed, resulting in ΔG>0 and an increase in the reaction rate.
B Because ΔG<0 , the hydrolysis of ATP is not thermodynamically favorable. In cells, enzymes act as catalysts that decrease ΔH for the reaction, resulting in ΔG>0 and an increase in the reaction rate.
C Although the hydrolysis of ATP is thermodynamically favorable, without a catalyst the reaction occurs at a very slow rate because it has a small activation energy.
D Although the hydrolysis of ATP is thermodynamically favorable, without a catalyst the reaction occurs at a very slow rate because it has a large activation energy.
▶️Answer/Explanation
Ans:D
Based on the value of ΔG , the hydrolysis of ATP is thermodynamically favorable, and the equilibrium favors the formation of ADP and free phosphate \(P_i\). However, the reaction does not occur at an appreciable rate because it is under kinetic control due to its large activation energy. The catalyst provides an alternate reaction pathway that has a lower activation energy, thereby increasing the rate.
Question
\(CO(l)(g)+2H_{2}(g)\rightleftharpoons CH_{3}OH(g) \) \(\Delta H<0\)
The synthesis of \(CH_3\)OH(g) from CO(g) and \(H_2\)(g) is represented by the equation above. The value of \(K_{c}\) for the reaction at 483 K is 14.5.
Which of the following statements is true about bond energies in this reaction?
(A) The energy absorbed as the bonds in the reactants are broken is greater than the energy released as the bonds in the product are formed.
(B) The energy released as the bonds in the reactants are broken is greater than the energy absorbed as the bonds in the product are formed.
(C) The energy absorbed as the bonds in the reactants are broken is less than the energy released as the bonds in the product are formed.
(D) The energy released as the bonds in the reactants are broken is less than the energy absorbed as the bonds in the product are formed.
▶️Answer/Explanation
Ans:C
Question
\(N_{2}(g) + 3 H_{2}(g) \rightleftharpoons 2 NH_{3}(g)\)
\(\Delta H^{\circ}{298}=-92 kJ/mol_{rxn}\) \(\Delta G^{\circ}_{298}=-33 kJ/mol{rxn}\)
Consider the reaction represented above at 298 K. When equal volumes of \(N_2(g)\) and \(H_{2}(g)\), each at 1 atm, are mixed in a closed container at 298 K, no formation of \(NH_{3}\)(g) is observed. Which of the following best explains the observation?
(A) The\( N_{2}\)(g) and the \(H_2\)(g) must be mixed in a 1:3 ratio for a reaction to occur.
(B) A high activation energy makes the forward reaction extremely slow at 298 K.
(C) The reaction has an extremely small equilibrium constant, thus almost no product will form.
(D) The reverse reaction has a lower activation energy than the forward reaction, so the forward reaction does not occur.
▶️Answer/Explanation
Ans:B
