Question
NO(g) + \(NO_3\)(g) → 2 \(NO_2\)(g)
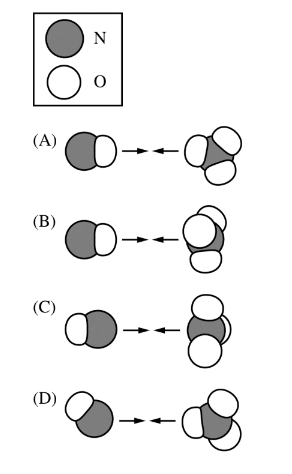
The reaction between NO(g) and \(NO_3\)(g) is represented by the equation above. Which of the following orientations of collision between NO(g) and \(NO_3\)(g) is most likely to be effective?
▶️Answer/Explanation
Ans:D
For the reaction between NO(g) and \(NO_3(g)\) to produce \(2NO_2(g)\), the most effective collision orientation would be one that allows the reactants to form the products most efficiently. This typically involves the approach of reactive parts of the molecules towards each other.
Based on the image description, here are the considerations for each orientation:
Orientation A: The nitrogen atom of NO colliding with an oxygen atom of \(NO_3\) might not be the most effective because it doesn’t directly lead to the formation of \(NO_2\).
Orientation B: The oxygen atom of NO colliding with an oxygen atom of \(NO_3\) is also less likely to be effective for the same reason as A.
Orientation C: The nitrogen atom of NO colliding with the nitrogen atom of \(NO_3\) could be effective if it leads to the formation of two \(NO_2\) molecules.
Orientation D: The oxygen atom of NO colliding with the nitrogen atom of \(NO_3\) seems the most likely to be effective, as it could facilitate the transfer of an oxygen atom to form two \(NO_2\) molecules.
Therefore, Orientation D is most likely to be the effective collision orientation for this reaction, as it aligns with the expected product formation.
Questions
\(Cl^{-}(aq)+ClO^{-}(aq)+2H^{+}(aq)\rightarrow Cl_{2}+H_{2}O_{(l)}\)
What effect will increasing [H+] at constant temperature have on the reaction represented above?
(A) The activation energy of the reaction will increase.
(B) The activation energy of the reaction will decrease.
(C) The frequency of collisions between H+(aq) ions and CIO-(aq) ions will increase.
(D) The value of the rate constant will increase.
▶️Answer/Explanation
Ans: C
The reaction is a redox reaction where chloride ions (\(Cl^-\)) and hypochlorite ions (\(ClO^-\)) react with hydrogen ions (\(H^+\)) to produce chlorine gas (\(Cl_2\)) and water (\(H_2O\)). This reaction is typically referred to as the disproportionation of hypochlorite.
To analyze the effect of increasing \([H^+]\) (concentration of hydrogen ions) at constant temperature on the reaction, we need to consider its mechanism:
1. \(ClO^-(aq) + 2H^+(aq) \rightarrow Cl_{2(g)} + H_{2(g)}O\)
(This represents the reduction half-reaction where \(ClO^-\) is reduced to \(Cl_2\).)
2. \(Cl^-(aq) + H^+(aq) \rightarrow \frac{1}{2}Cl_{2(g)} + \frac{1}{2}H_{2(g)}O\)
(This represents the oxidation half-reaction where \(Cl^-\) is oxidized to \(Cl_2\).)
The rate of a reaction depends on the frequency of successful collisions between reactant molecules. Increasing the concentration of \(H^+\) ions would increase the frequency of collisions between \(H^+\) ions and \(ClO^-\) ions, as per the first half-reaction. Thus, option (C) is correct: The frequency of collisions between \(H^+(aq)\) ions and \(ClO^-(aq)\) ions will increase.
Option (A) and (B) are related to the activation energy, but changing the concentration of \(H^+\) ions does not directly affect the activation energy of the reaction.
Option (D) is incorrect because while increasing the concentration of reactants typically increases the rate constant, it doesn’t directly relate to the rate of the reaction in this case. The rate constant depends on other factors like temperature and the nature of the reactants.
Question
Reaction A : \(O+O→O_2\)
Reaction B : \(C_2H_4+C_2H_4→C_4H_8\)
Reaction C : \(CO+O_2→CO_2+O\)
Reaction D : \(CH_3I+Br^−→CH_3Br+I^−\)
The equations shown above represent four elementary reactions. Which of the following identifies the reaction in which the number of successful collisions and reaction rate are independent of the orientation of the reactants and explains why?
A Reaction A , because the electron clouds of the O atoms are distributed symmetrically.
B Reaction B , because each \(C_2H_4\) molecule has a double bond.
C Reaction C , because both CO and \(O_2\) are linear molecules.
D Reaction D , because the reactant \(Br^−\) and the product \(I^−\) are negatively charged.
▶️Answer/Explanation
Ans:A
The electron cloud is distributed symmetrically around the O atoms; thus the orientation of the two reacting O atoms during a collision is not a factor in determining the success of the collision and the reaction rate.
Question
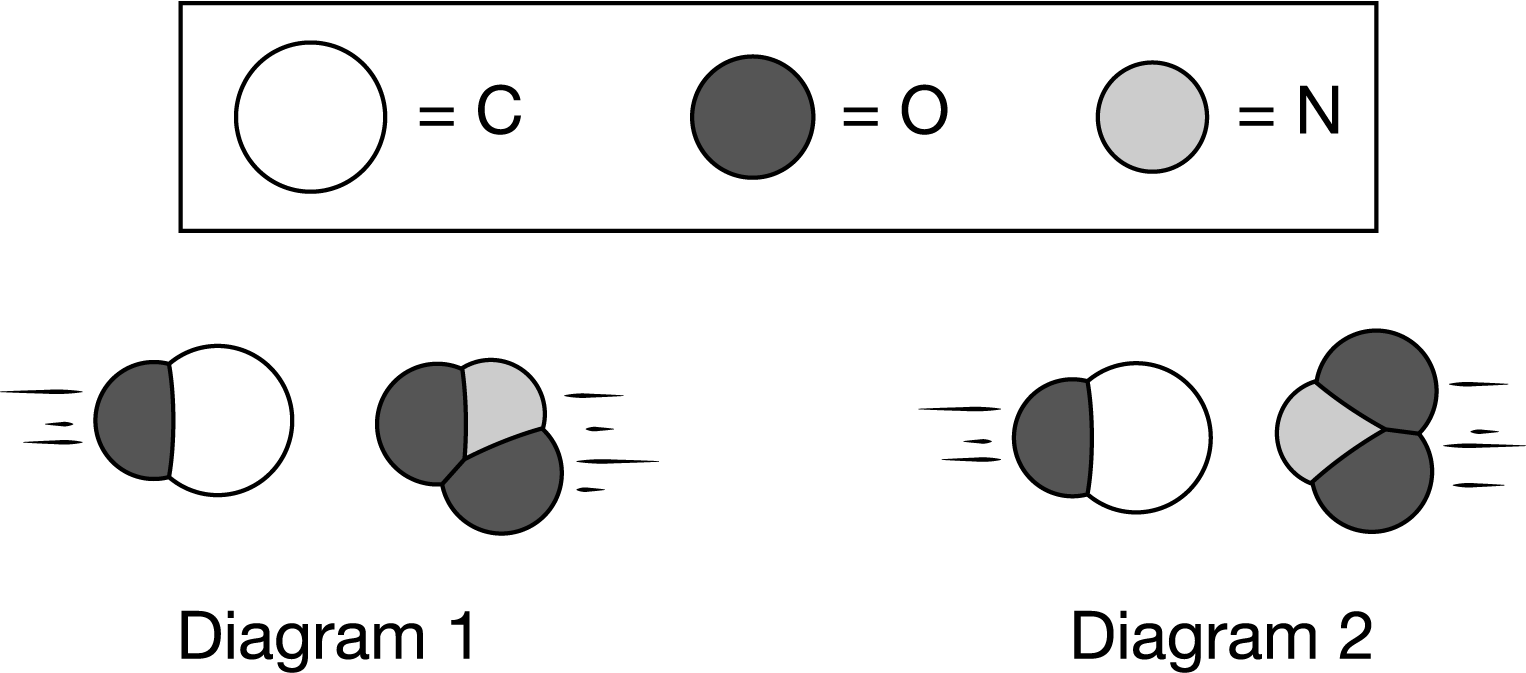
The two diagrams above represent collisions that take place at the same temperature between a CO molecule and an \(NO_2\) molecule. The products are \(CO_2\) and NO. Which diagram most likely represents an effective collision, and why?
A Diagram 1 represents an effective collision because the molecules have about the same size and the same energy, which leads to a larger rate constant (k).
B Diagram 1 represents an effective collision because the two molecules have the proper orientation to form a new C−O bond as long as they possess enough energy to overcome the activation energy barrier.
C Diagram 2 represents an effective collision because the molecules are aligned head-to-head and that maximizes the overlap between their atomic orbitals, forming more products.
D Diagram 2 represents an effective collision because the oxygen atoms are the most electronegative and will minimize their repulsions when oriented away from each other.
▶️Answer/Explanation
Ans: B
In order for this reaction to form \(CO_2\) and NO, the reactants CO and \(NO_2\) must be oriented in a way that allows for a bond between N and O to be broken while a new bond between C and O forms. Diagram 1 represents the best orientation for an effective collision as long as the molecules have the minimum energy required to overcome the activation energy barrier.
Question
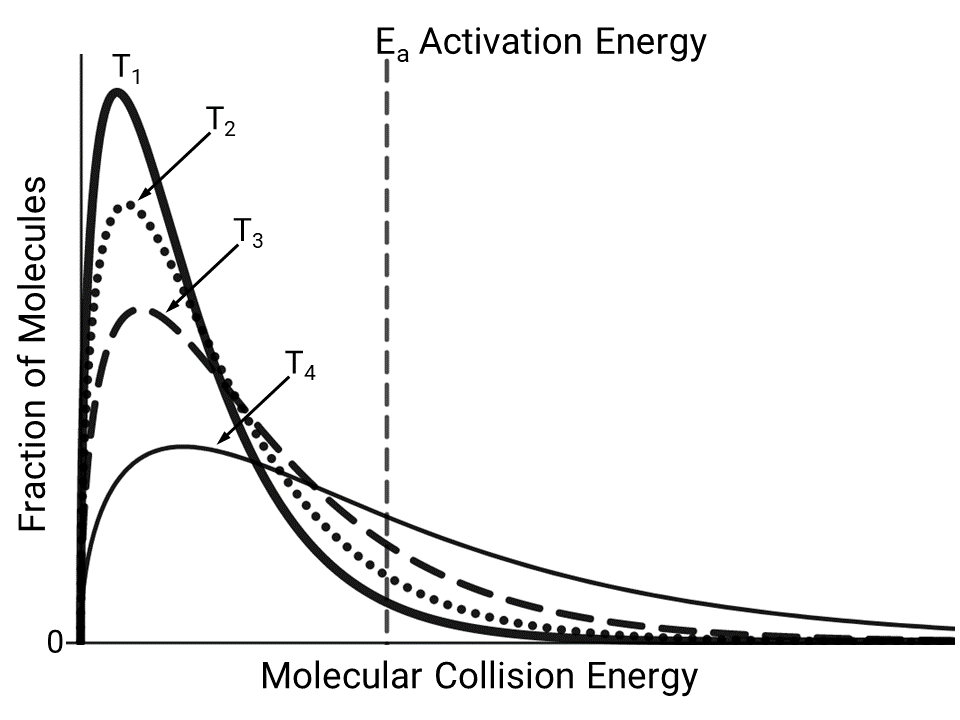
The diagram above shows the distribution of molecular collision energies for equimolar samples of a reactant at different temperatures. Based on the diagram, at which temperature will the reactant be consumed at the fastest rate, and why?
A At \(T_1\), because a larger fraction of the molecules have about the same energy.
B At \(T_2\), because at this temperature most of the molecules undergo collisions frequently.
C At \(T_3\), because at this temperature the rate of consumption for about half of the molecules is determined by their orientation.
D At \(T_4\), because a larger fraction of the molecules have an energy that is equal to or greater than the activation energy.
▶️Answer/Explanation
Ans: D
The rate of consumption of a reactant depends on temperature, and for most reactions an increase in temperature increases the rate. This is a result of an increase in the number of molecules whose energy is equal to or greater than the minimum energy required to overcome the activation energy \(E_a\) barrier. This is shown in the energy distribution corresponding to T4 because a larger portion of the curve is located at energies greater than or equal to \(E_a\).
Question
\(NO(g) + NO_{3}(g) → 2 NO_{2}(g)\)
\(rate = k[NO][NO_{3}]\)
The reaction represented above occurs in a single step that involves the collision between a particle of NO and a particle of \(NO_3\). A scientist correctly calculates the rate of collisions between NO and\( NO_3\) that have sufficient energy to overcome the activation energy. The observed reaction rate is only a small fraction of the calculated collision rate. Which of the following best explains the discrepancy?
(A) The energy of collisions between two reactant particles is frequently absorbed by collision with a third particle.
(B) The two reactant particles must collide with a particular orientation in order to react.
(C) The activation energy for a reaction is dependent on the concentrations of the reactant particles.
(D) The activation energy for a reaction is dependent on the temperature.
▶️Answer/Explanation
Ans:B