Question
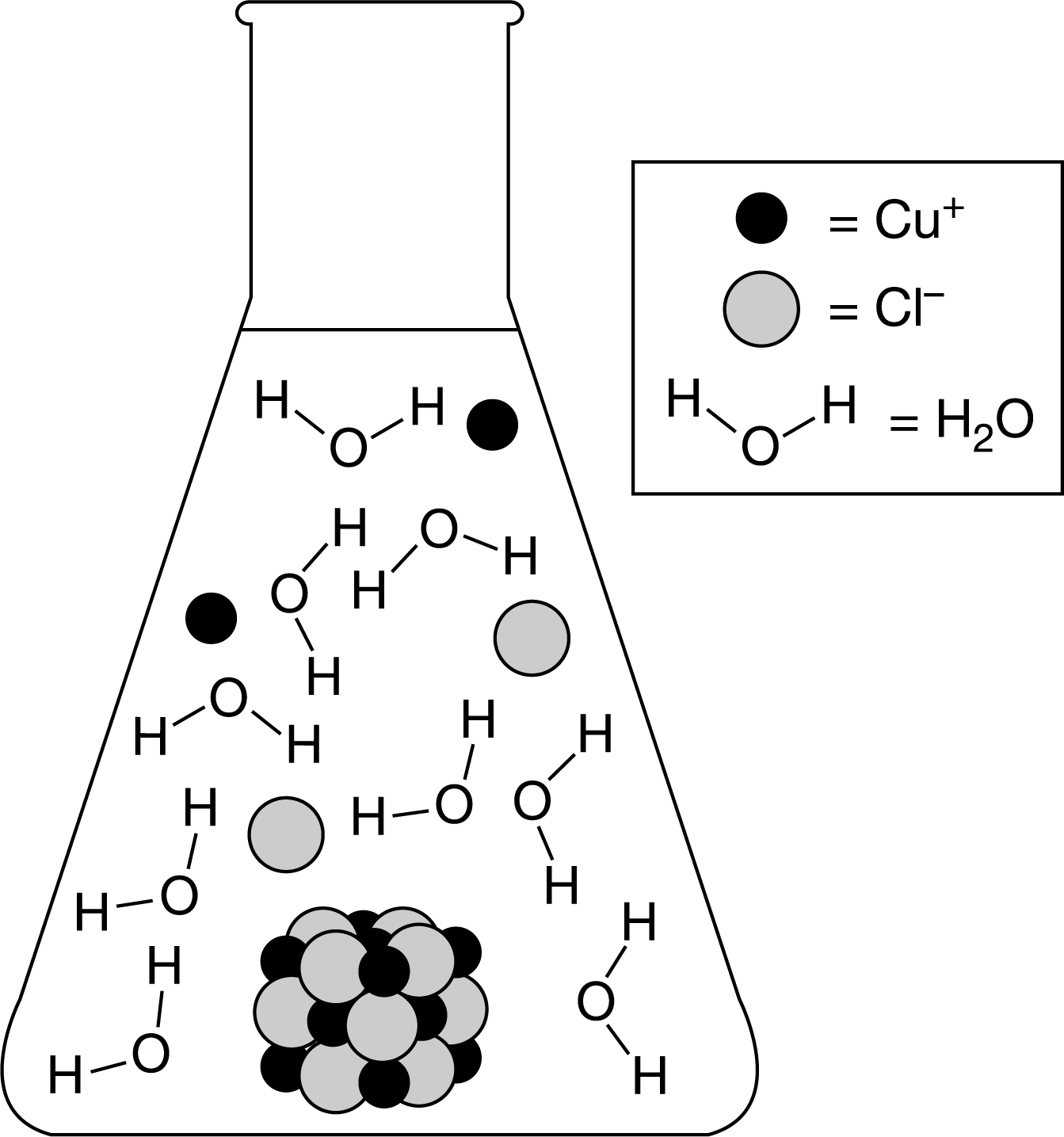
The particle diagram shown above represents the dissolution of \(CuCl(s)\) assuming an equilibrium concentration for \(Cu^+\) ions of about \(4\times 10^{−4}\) M in a saturated solution at 25°C. The equilibrium being represented is shown in the following chemical equation.
\(CuCl\)(s)⇄\(Cu^+(aq)+Cl^−(aq)\)
Which of the following changes to the particle diagram will best represent the effect of adding 1.0mL of 4M \(NaCl\) to the solution?
A Some of the \(Cu^+\) and \(Cl^−\) ions combine to form \(CuCl\)(s) because the \(K_{sp}\) will be lower than \(1.6\times 10^{−7}\) .
B Some of the \(Cu^+\) and \(Cl^−\) ions combine to form \(CuCl\)(s) because the molar solubility will be lower than \(4\times 10^{−4}\)M .
C More \(Cu^+\) and \(Cl^−\) ions will be in solution because the molar solubility will be higher than \(4\times 10^{−4}\)M .
D More \(Cu^+\) and \(Cl^−\) ions will be in solution because the \(K_{sp}\) will be higher than \(1.6\times 10^{−7}\) .
▶️Answer/Explanation
Ans:B
The increase in the concentration of \(Cl^−\) ions in solution with the addition of \(NaCl\) will disrupt the equilibrium, the rate of the reverse reaction will increase, and the system will reach a new equilibrium position. As a result, \(Cu^+\) and \(Cl^−\) ions will combine to form \(CuCl\)s) and the molar solubility will be lower than \(4\times 10^{−4}\)M.
Question
\(Ca(OH)_2\)(s)⇄\(Ca^{2+}(aq)+2OH^−\)(aq) \(K_{sp}=5.5\times 10^{−6}\) at298K
The equilibrium in a saturated solution of \(Ca(OH)_2\) is represented above. In an experiment, a student places 5.0g of \(Ca(OH)_2\)(s) into 100.0mL of distilled water and stirs the mixture. How would the results be affected if the student repeats the experiment but this time places 5.0g of \(Ca(OH)_2\)(s) into 100.0mL of 0.0010M \(NaOH\)(aq) instead of distilled water?
A Less solid will dissolve, because the larger value of \([OH^−]\) will cause the equilibrium position to lie farther to the right.
B Less solid will dissolve, because the larger value of \([OH^−]\) will cause the equilibrium position to lie farther to the left.
C More solid will dissolve, because the larger value of \([OH^−]\) will cause the equilibrium position to lie farther to the right.
D More solid will dissolve, because the smaller value of \([OH^−]\) will cause the equilibrium position to lie farther to the left.
▶️Answer/Explanation
Ans:B
When \(Ca(OH)_2\)(s) starts to dissolve in the \(NaOH\)(aq) solution, the presence of the \(OH^−\)(aq) ions already in the solution leads to more frequent collisions between \(Ca^{2+}\)(aq) and \(OH^−\)(aq), which increases the rate of the reverse (i.e., precipitation) reaction. An equilibrium becomes established but with less of the \(Ca(OH)_2\)(s) dissolving than in the first experiment.
Question
\(AgCN(s)\)⇄\(Ag^+(aq)+CN^−(aq)\)
The dissolution of solid \(AgCN\) is represented by the chemical equation above. In pure water, the equilibrium concentration of \(Ag^+\) ions in a saturated solution is \(7.7\times 10^{−9}\) M. If a small amount of solid \(NaCN\) is added to the saturated \(AgCN\) solution, which of the following would be observed?
A The \(K_{sp}\) increases and more \(AgCN\) dissolves.
B The \(K_{sp}\) increases and some \(AgCN\) precipitates.
C The molar solubility of \(AgCN\) becomes smaller than \(7.7\times 10^{−9}\)M and some \(AgCN\) precipitates.
D The molar solubility of \(AgCN\) becomes larger than \(7.7\times 10^{−9}\)M and more \(AgCN\) dissolves.
▶️Answer/Explanation
Ans:C
Adding \(CN^−\) ions decreases the molar solubility of \(AgCN\) by increasing the rate at which the reverse reaction in the dissolution equilibrium occurs, causing the precipitation of some \(AgCN\).
Question
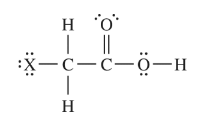
The structure of haloacetic acids, \(XCH_{2}COOH\) (where X is either F, Cl, Br, or I ), is shown above. The dissociation constants and
molar masses of four haloacetic acids are listed in the table below.
An aqueous solution contains small but equal concentrations of both chloroacetic and fluoroacetic acids. Which statement comparing the percent ionizations of the two acids in the solution is true?
(A) The percent ionization of chloroacetic acid is greater than that of fluoroacetic acid.
(B) The percent ionization of chloroacetic acid is less than that of fluoroacetic acid.
(C) The percent ionizations cannot be compared without knowing the concentrations of the two acids.
(D) The percent ionizations cannot be compared without knowing the pH of the solution.
▶️Answer/Explanation
Ans:C
