Question
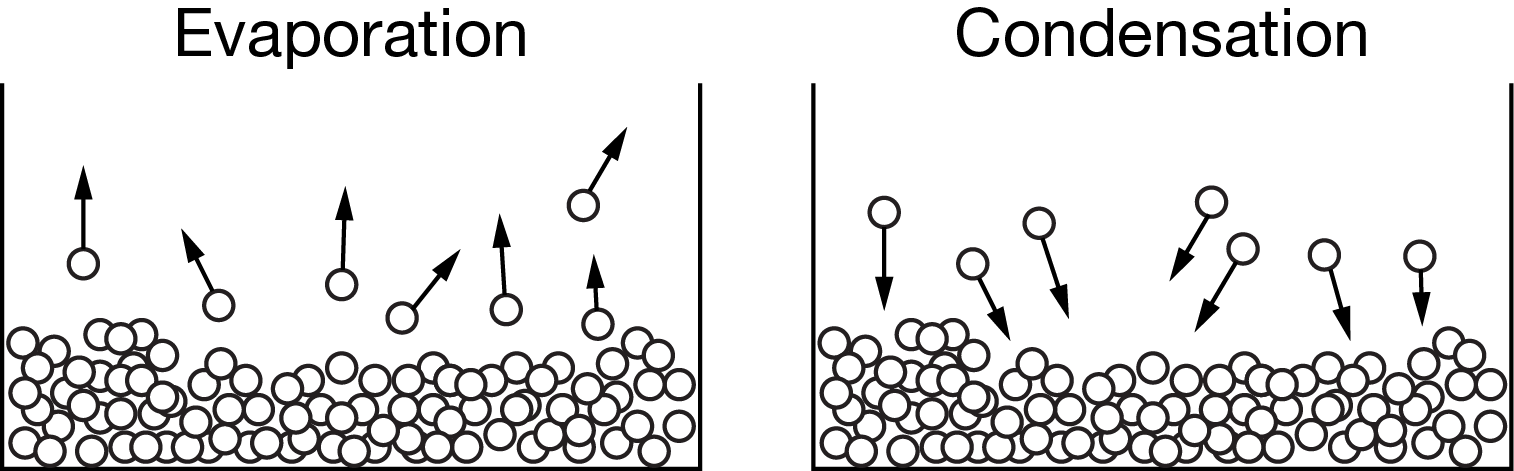
Particulate models of the evaporation of liquid water and the condensation of water vapor are shown above. Based on these models, which of the following accurately compares the energy changes associated with each of the phase changes?
A The amount of energy absorbed by one mole of water molecules as they escape the liquid is greater than the amount of energy released by one mole of water molecules as they come together to form a liquid.
B The amount of energy absorbed by one mole of water molecules as they escape the liquid is less than the amount of energy released by one mole of water molecules as they come together to form a liquid.
C The amount of energy absorbed by one mole of water molecules as they escape the liquid is equal to the amount of energy released by one mole of water molecules as they come together to form a liquid.
D The amount of energy absorbed by one mole of water molecules escaping the liquid can be either greater than or less than the amount of energy released by one mole of water molecules as they come together to form a liquid.
▶️Answer/Explanation
Ans: C
The amount of energy either absorbed or released during complementary phase changes is constant.
Question
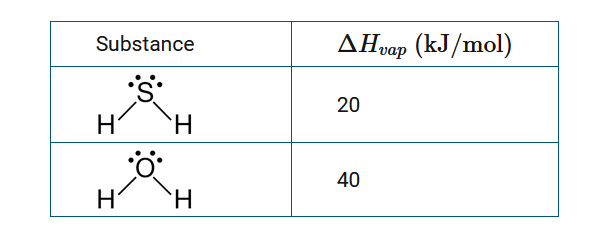
The table above provides the values for some physical properties of \(H_2S\) and \(H_2O\). Approximately, how many moles of \(H_2S\) must be condensed to release as much heat as would be released when 1 mole of \(H_2O\) is condensed?
A 0.5 mole of \(H_2S\)
B 1 mole of \(H_2S\)
C 2 moles of \(H_2S\)
D 4 moles of \(H_2S\)
▶️Answer/Explanation
Ans:C
Since \(\Delta H_{vap}\) of \(H_2O\) is approximately \(2\times \Delta H_{vap}\) of \(H_2S\), two moles of \(H_2S\) must be condensed to release the same amount of heat as the condensation of 1 mole of \(H_2O\).
Question
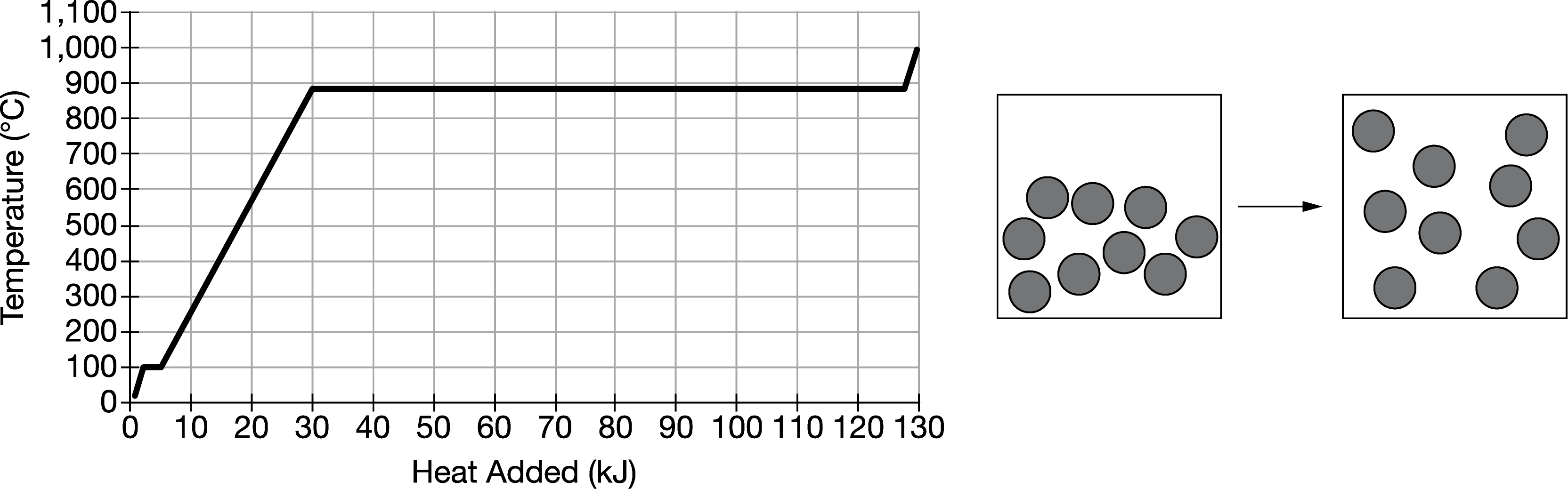
The heating curve for 1.0 mole of Na, initially at 25.0°C, is shown above at the left. Which of the following best explains the change in heat when 0.50 mole of Na undergoes the transition shown in the diagram above to the right?
A Approximately 13kJ of heat are absorbed as a result of the increase in potential energy between the Na atoms.
B Approximately 49kJ of heat are absorbed to overcome the attractive forces acting between Na atoms.
C Approximately 98kJ of heat are released as a result of the decrease in the kinetic energy of the Na atoms.
D Approximately 120kJ of heat are released to decrease the potential energy between Na atoms.
▶️Answer/Explanation
Ans:B
The diagram represents the transition Na(l)→Na(g) . In the heating curve, the horizontal segment seen at a temperature close to 880°C represents the amount of heat absorbed during the liquid-to-gas phase transition for 1.0 mole of Na, which is approximately 98kJ. For 0.50 mole of Na, only half of this amount of heat is needed, approximately 49kJ.
Question
The direct conversion of a solid to a gas is called __________.
A) fusion B) sublimation C) condensation D) boiling E) vaporization
▶️Answer/Explanation
Ans: B
Correct answer: (b). Be familiar with the conversion of dry ice, which is \(CO_2\) (s), to \(CO_2\) (g). Know the names of all
phase changes (solid to liquid, liquid to gas, etc). Know whether energy (heat) is released or absorbed by the
system for all phase changes.
Question
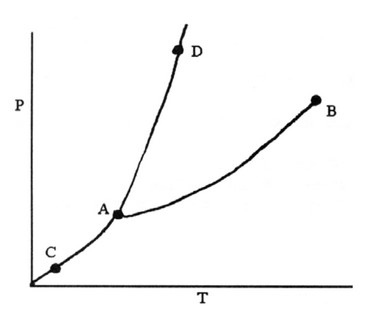
On the phase diagram above, segment __________ corresponds to the conditions of temperature and pressure
under which the solid and the gas of the substance are in equilibrium.
A) CD B) AB C) AD D) BC E) AC
▶️Answer/Explanation
Ans: E
Segment AC [answer choice (e)] is correct. Be sure to memorize the regions of the phase diagram (solid, liquid
gas).
