Question
\(CaCO_{3}(s)\rightleftharpoons CaO(s)+CO_{2}(g)\)
When heated, calcium carbonate decomposes according to the equation above. In a study of the decomposition of calcium carbonate, a student added a 50.0 g sample of powdered \(CaCO_{3}\)(s) to a 1.00 L rigid container. The student sealed the container, pumped out all the gases, then heated the container in an oven at 1100 K. As the container was heated, the total pressure of the \(CO_2\)(g) in the container was measured over time. The data are plotted in the graph below.
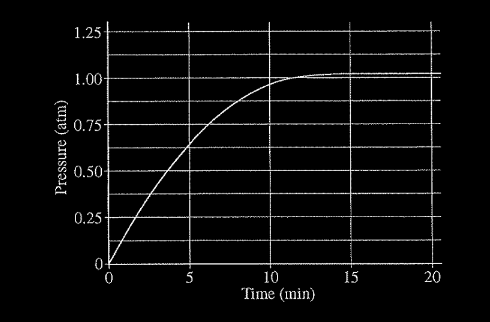
The student repeated the experiment, but this time the student used a 100.0 g sample of powdered \(CaCO_3\)(s). In this experiment, the final pressure in the container was 1.04 atm, which was the same final pressure as in the first experiment.
(a) Calculate the number of moles of \(CO_{2}(g)\) present in the container after 20 minutes of heating.
(b) The student claimed that the final pressure in the container in each experiment became constant because all of the\( CaCO_{3}\)(s) had decomposed. Based on the data in the experiments, do you agree with this claim? Explain.
(c) After 20 minutes some \(CO_2\)(g) was injected into the container, initially raising the pressure to 1.5 atm. Would the final pressure inside the container be less than, greater than, or equal to 1.04 atm? Explain your reasoning.
(d) Are there sufficient data obtained in the experiments to determine the value of the equilibrium constant, K,for the decomposition of \(CaCO_3\)(s) at 1100 K? Justify your answer.
▶️Answer/Explanation
(a) \(PV=nRT\)
\(\frac{PV}{RT}=n=\frac{(1.04 atm)(1.00 L)}{(0.0821\frac{L atm}{mol K})(1100 K)}== 0.0115 mol CO_2\)
(b) Do not agree with claim
Explanation I: In experiment 1, the moles of \(CaCO_3 = 50.0 g/100.09 g/mol = 0.500 mol CaCO_3\). If the reaction had gone to completion, 0.500 mol of \(CO_2\) would have been produced. From part (a) only 0.0115 mol was produced. Hence, the student’s claim was false.
Explanation II: The two different experiments (one with 50.0 g of \(CaCO_3\) and one with 100.0 g of \(CaCO_3\)) reached the same constant, final pressure of 1.04 atm. Since increasing the amount of reactant did not produce more product, there is no way that all of the \(CaCO\), reacted. Instead, an equilibrium condition has been achieved and there must be some solid \(CaCO_3\) in the container.
(c) The final pressure would be equal to 1.04 atm. Equilibrium was reached in both experiments; the equilibrium pressure at this temperature is 1.04 atm. As the reaction shifts toward the reactant, the amount of \(CO_2\)(g) in the container will decrease until the pressure returns to 1.04 atm.
(d) Yes. For the equilibrium reaction represented by the chemical equation in this problem, at a given temperature the equilibrium pressure of \(CO_2\) determines the equilibrium constant. Since the measured pressure of \(CO_2\) is also the equilibrium pressure of \(CO_2\), \(K_p= P_{co_2} =1.04\).
Note: If the response in part (b) indicates “yes”, that all of the \(CaCO_3\)(s) had decomposed, then the point can be earned by stating that the system did not reach equilibrium in either experiment and hence the value of \(K_p\), cannot be calculated from the data.
