Question
A student has 100. mL of 0.400 M \(CuSO_4(aq)\) and is asked to make 100. mL of 0.150 M \(CuSO_4\)(aq) for a spectrophotometry experiment. The following laboratory equipment is available for preparing the solution: centigram balance, weighing paper, funnel, 10 mL beaker, 150 mL beaker, 50 mL graduated cylinder, 100 mL volumetric flask, 50 mL buret, and distilled water.
(a) Calculate the volume of 0.400 M\( CuSO_4\)(aq) required for the preparation.
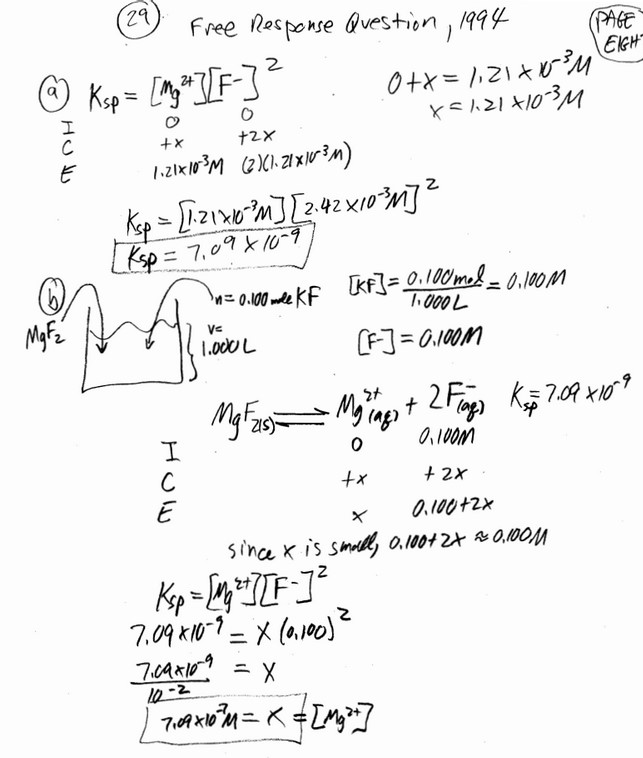
(b) Briefly describe the essential steps to most accurately prepare the 0.150 M \(CuSO_4\)(aq) from the 0.400 M \(CuSO_4\)(aq) using the equipment listed above. The student plans to conduct a spectrophotometric analysis to determine the concentration of \(Cu^{2+}\)(aq) in a solution. The solution has a small amount of \(Co(NO_3)_2\)(aq) present as a contaminant. The student is given the diagram below, which shows the absorbance curves for aqueous solutions of \(Co^{2+}\)(aq) and\( Cu^{2+}\)(aq).
(c) The spectrophotometer available to the student has a wavelength range of 400 nm to 700 nm. What wavelength should the student use to minimize the interference from the presence of the \(Co^{2+}\)(aq) ions?
▶️Answer/Explanation
(a) \(M_1V_{1}=M_{2}V_{2}\)
\(V_{2}=\frac{(0.150M)(0.100L)}{0.400M}\)
\(V_{2}=0.0375L \times \frac{1000mL}{1L}=37.5mL\)
(b) Use the buret to dispense 37.5 mL of\( CuSO_4\) solution into the volumetric flask. Fill to the mark with distilled water.
(c) 700 nm (Any wavelength from 650 to 700 nm is acceptable.)
Question
Answer the following questions relating to HCl,\(CH_3Cl,\) and \(CH_3\)Br.
(a) HCl(g) can be prepared by the reaction of concentrated \(H_ 2SO_4\)(aq) with NaCl(s), as represented by the following equation.
\(H_{2}SO_{4}(aq)+2NaCl(s)\rightarrow 2HCl(g)+Na_{2}SO_{4}(aq)\)
(i) A student claims that the reaction is a redox reaction. Is the student correct? Justify your answer.
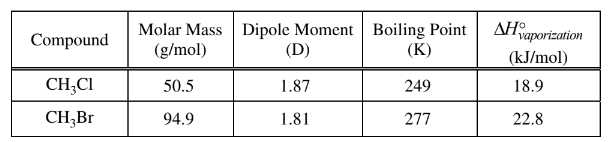
(ii) Calculate the mass, in grams, of NaCl(s) needed to react with excess \(H_2SO_4\)(aq) to produce 3.00 g of HCl(g). Assume that the reaction goes to completion.HCl(g) can react with methanol vapor,\( CH_3OH(g)\), to produce \(CH_3Cl\)(g), as represented by the following equation.
\(CH_{3}OH(g)+HCl(g)\rightleftharpoons CH_{3}Cl(g)+H_{2}O(g) K_{P}=4.7\times 10^{3}\) At400K
(b) \(CH _3OH(g)\) and HCl(g) are combined in a 10.00 L sealed reaction vessel and allowed to reach equilibrium at 400 K. The initial partial pressure of\( CH_3OH(g)\) in the vessel is 0.250 atm and that of HCl(g) is 0.600 atm.
(i) Does the total pressure in the vessel increase, decrease, or remain the same as equilibrium is approached? Justify your answer in terms of the reaction stoichiometry.
(ii) Considering the value of Kp , calculate the final partial pressure of HCl(g) after the system inside the vessel reaches equilibrium at 400 K.
(iii) The student claims that the final partial pressure of \(CH_3OH(g) \)at equilibrium is very small but not exactly zero. Do you agree or disagree with the student’s claim? Justify your answer.
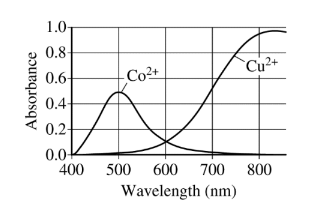
(c) The table below shows some data for the compounds \(CH _3Cl \)and \(CH_3Br\).
(i) Identify all the types of intermolecular forces that exist among molecules in \(CH_3Cl(l)\).
(ii) In terms of intermolecular forces, explain why the boiling point of CH3Br(l) is greater than that of \(CH_3Cl(l)\).
(d) A 2.00 mL sealed glass vial containing a 1.00 g sample of \(CH_3Cl(l)\) is stored in a freezer at 233 K.
(i) Calculate the pressure in the vial at 298 K assuming that all the \(CH_3Cl(l)\)vaporizes.
(ii) Explain why it would be unsafe to remove the vial from the freezer and leave it on a lab bench at 298 K.
▶️Answer/Explanation
a(i) No, the student is not correct. None of the oxidation numbers of the elements change (H = +1, S = +6, O = -2, Na = +1, Cl = -1).
a(ii) 
(b)(i) The pressure will remain the same. The reaction stoichiometry shows that two moles of gaseous reactants produce two moles of gaseous products. Because the number of moles of gas does not change, the pressure does not change.
b(ii)The value of\( K_p\) is large, so the reaction will proceed to the right until the limiting reactant is essentially used up. Thus practically all of the \(CH_3OH(g)\) will react and the final pressure of HCl(g) is 0.600 – 0.250 = 0.350 atm.
OR
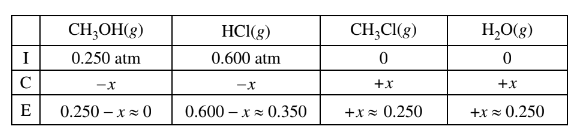
The final pressure of HCl(g) is 0.350 atm at equilibrium.
b(iii) Agree. The large value of \(K_p\) means that the partial pressure of the limiting reactant at equilibrium will be extremely small, but some \(CH_3OH \)molecules must exist for the system to be in dynamic equilibrium.
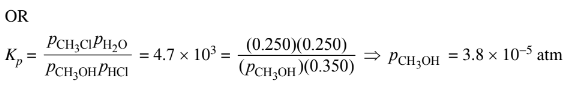
The partial pressure of \(CH_3OH(g)\) is very small but is not zero.
c(i) London dispersion forces and dipole-dipole forces
c(ii) The electron cloud in \(CH_3Br\) is larger and more polarizable than that of \(CH_3Cl\). As a result the London dispersion forces are stronger in \(CH_3Br\) compared to those in \(CH_3Cl\)and consequently the boiling point of\( CH_3\)Br is higher than that of \(CH_3Cl\).
d(i)
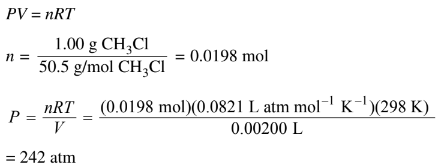
d(ii) At room temperature the liquid will vaporize. Consequently the glass vial may not be strong enough to withstand the increase in pressure.
Question
\(MgF_2(s) \Leftrightarrow Mg^{2+}(aq) + 2 F^{ -}(aq)\)
In a saturated solution of \(MgF_2\) at 18° C, the concentration of Mg2+ is \(1.21 x 10^ {-3}\) molar. The equilibrium is represented by the equation above.
(a) Write the expression for the solubility-product constant, \(K_{sp}\), and calculate its value at 18° C.
(b) Calculate the equilibrium concentration of \(Mg^{2+}\) in 1.000 liter of saturated \(MgF_2\) solution at 18°C to which 0.100 mole of solid KF has been added. The KF dissolves completely. Assume the volume change is negligible.
(c) Predict whether a precipitate of \(MgF_2\) will form when 100.0 milliliters of a \(3.00 x 10 ^{-3}\) molar \(Mg(NO_3)_2\) solution is mixed with 200.0 milliliters of a \(2.00 x 10 ^{-3}\) molar NaF solution at 18°C.
Calculations to support your prediction must be shown.
(d) At 27°C the concentration of \(Mg^{2+}\) in a saturated solution of \(MgF_2\) is \(1.17 x 10 ^{-3}\) molar. Is the dissolving of \(MgF_2\) in water an endothermic or an exothermic process? Give an explanation to support
your conclusion.
▶️Answer/Explanation
Answer: