Question
A student has 100. mL of 0.400 M \(CuSO_4(aq)\) and is asked to make 100. mL of 0.150 M \(CuSO_4\)(aq) for a spectrophotometry experiment. The following laboratory equipment is available for preparing the solution: centigram balance, weighing paper, funnel, 10 mL beaker, 150 mL beaker, 50 mL graduated cylinder, 100 mL volumetric flask, 50 mL buret, and distilled water.
(a) Calculate the volume of 0.400 M\( CuSO_4\)(aq) required for the preparation.
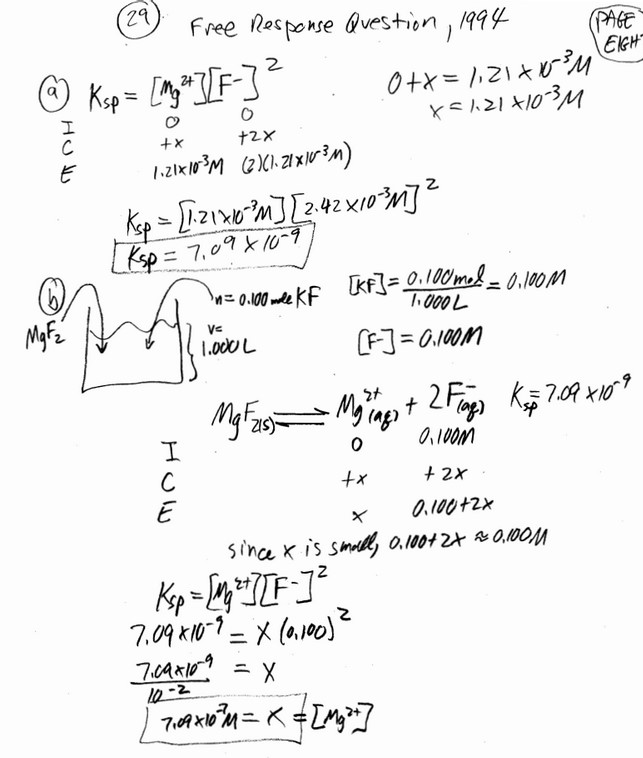
(b) Briefly describe the essential steps to most accurately prepare the 0.150 M \(CuSO_4\)(aq) from the 0.400 M \(CuSO_4\)(aq) using the equipment listed above. The student plans to conduct a spectrophotometric analysis to determine the concentration of \(Cu^{2+}\)(aq) in a solution. The solution has a small amount of \(Co(NO_3)_2\)(aq) present as a contaminant. The student is given the diagram below, which shows the absorbance curves for aqueous solutions of \(Co^{2+}\)(aq) and\( Cu^{2+}\)(aq).
(c) The spectrophotometer available to the student has a wavelength range of 400 nm to 700 nm. What wavelength should the student use to minimize the interference from the presence of the \(Co^{2+}\)(aq) ions?
▶️Answer/Explanation
(a) \(M_1V_{1}=M_{2}V_{2}\)
\(V_{2}=\frac{(0.150M)(0.100L)}{0.400M}\)
\(V_{2}=0.0375L \times \frac{1000mL}{1L}=37.5mL\)
(b) Use the buret to dispense 37.5 mL of\( CuSO_4\) solution into the volumetric flask. Fill to the mark with distilled water.
(c) 700 nm (Any wavelength from 650 to 700 nm is acceptable.)
Question
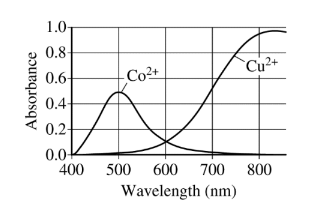
\(MgF_2(s) \Leftrightarrow Mg^{2+}(aq) + 2 F^{ -}(aq)\)
In a saturated solution of \(MgF_2\) at 18° C, the concentration of Mg2+ is \(1.21 x 10^ {-3}\) molar. The equilibrium is represented by the equation above.
(a) Write the expression for the solubility-product constant, \(K_{sp}\), and calculate its value at 18° C.
(b) Calculate the equilibrium concentration of \(Mg^{2+}\) in 1.000 liter of saturated \(MgF_2\) solution at 18°C to which 0.100 mole of solid KF has been added. The KF dissolves completely. Assume the volume change is negligible.
(c) Predict whether a precipitate of \(MgF_2\) will form when 100.0 milliliters of a \(3.00 x 10 ^{-3}\) molar \(Mg(NO_3)_2\) solution is mixed with 200.0 milliliters of a \(2.00 x 10 ^{-3}\) molar NaF solution at 18°C.
Calculations to support your prediction must be shown.
(d) At 27°C the concentration of \(Mg^{2+}\) in a saturated solution of \(MgF_2\) is \(1.17 x 10 ^{-3}\) molar. Is the dissolving of \(MgF_2\) in water an endothermic or an exothermic process? Give an explanation to support
your conclusion.
▶️Answer/Explanation
Answer: