Questions
\(Cu_{(s)}+4HNO_{3}(aq)\rightarrow Cu(No_{3})_{2}(aq)+2No_{2(g)}+2H_{2}o_{(l)}\)
Each student in a class placed a \(2.00 \mathrm{~g}\) sample of a mixture of \(\mathrm{Cu}\) and \(\mathrm{Al}\) in a beaker and placed the beaker in a fume hood. The students slowly poured \(15.0 \mathrm{~mL}\) of \(15.8 \mathrm{M} \mathrm{HNO}_3(a q)\) into their beakers. The reaction between the copper in the mixture and the \(\mathrm{HNO}_3(a q)\) is represented by the equation above. The students observed that a brown gas was released from the beakers and that the solutions turned blue, indicating the formation of \(\mathrm{Cu}^{2+}(a q)\). The solutions were then diluted with distilled water to known volumes.
Which of the following is true about the reaction?
(A) It is a Brønsted-Lowry acid-base reaction, because the solution is neutral at the end.
(B) It is a Brønsted-Lowry acid-base reaction, because \(HNO_{3}(aq))\ is a strong acid.
(C) It is a redox reaction, because \(Cu_{(s)}\) is oxidized and H+(aq) is reduced.
(D) It is a redox reaction, because \(Cu_{(s)}\) is oxidized and the nitrogen atom in \( No_{3}^{-}(aq)\) is reduced.
▶️Answer/Explanation
Ans: D
The given reaction is:
\[ \text{Cu}_{(s)} + 4\text{HNO}_{3(\text{aq})} \rightarrow \text{Cu(NO}_3\text{)}_{2(\text{aq})} + 2\text{NO}_2\text{ (g)} + 2\text{H}_{2}\text{O}_{(\text{l})} \]
This is a redox (oxidation-reduction) reaction, where Cu(s) is oxidized, and the nitrogen atoms in \( \text{HNO}_3(\text{aq}) \) are reduced.
In this reaction:
\( \text{Cu(s)} \) is oxidized from the \( 0 \) oxidation state to \( +2 \) oxidation state (\( \text{Cu}^{2+} \)).
The nitrogen atoms in \( \text{HNO}_3(\text{aq}) \) are reduced from the \( +5 \) oxidation state to the \( +4 \) oxidation state in \( \text{NO}_2(\text{g}) \).
Therefore, the correct answer is (D) It is a redox reaction because \( \text{Cu}_{(s)} \) is oxidized, and the nitrogen atom in \( \text{NO}_3^{-}(\text{aq}) \) is reduced.
(A) and (B) are incorrect because this is not a Brønsted-Lowry acid-base reaction. While \( \text{HNO}_3(\text{aq}) \) is a strong acid, the reaction involves the transfer of electrons (oxidation and reduction), not just the transfer of protons (\( \text{H}^{+} \)).
(C) is incorrect because \( \text{H}^{+}(\text{aq}) \) is not reduced in this reaction. The reducing agent is the copper metal (\( \text{Cu}_{(s)} \)), and the oxidizing agent is the nitric acid (\( \text{HNO}_3(\text{aq}) \)).
Question
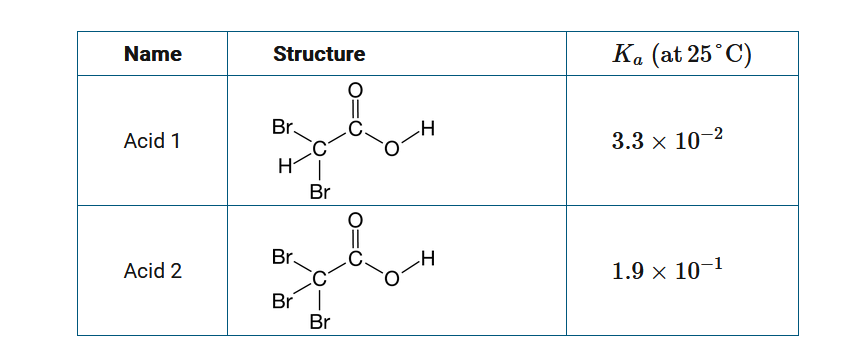
The table above provides information on two weak acids. Which of the following explains the difference in their acid strength?
A Acid 1 is a stronger acid because it has more acidic hydrogen atoms than acid 2.
B Acid 1 is a stronger acid because it can make more hydrogen bonds than acid 2.
C Acid 2 is a stronger acid because it is a larger, more polarizable molecule with stronger intermolecular forces than acid 1.
D Acid 2 is a stronger acid because it has a more stable conjugate base than acid 1 due to the greater number of electronegative Br atoms.
▶️Answer/Explanation
Ans:D
These two acids differ in the substitution of one H atom by a more electronegative Br atom, making the conjugate base \(C_2Br_3O_2^−\) a more stable (weaker) base compared to \(C_2HBr_2O_2^−\). The more stable a conjugate base is, the stronger the weak acid is.
Question
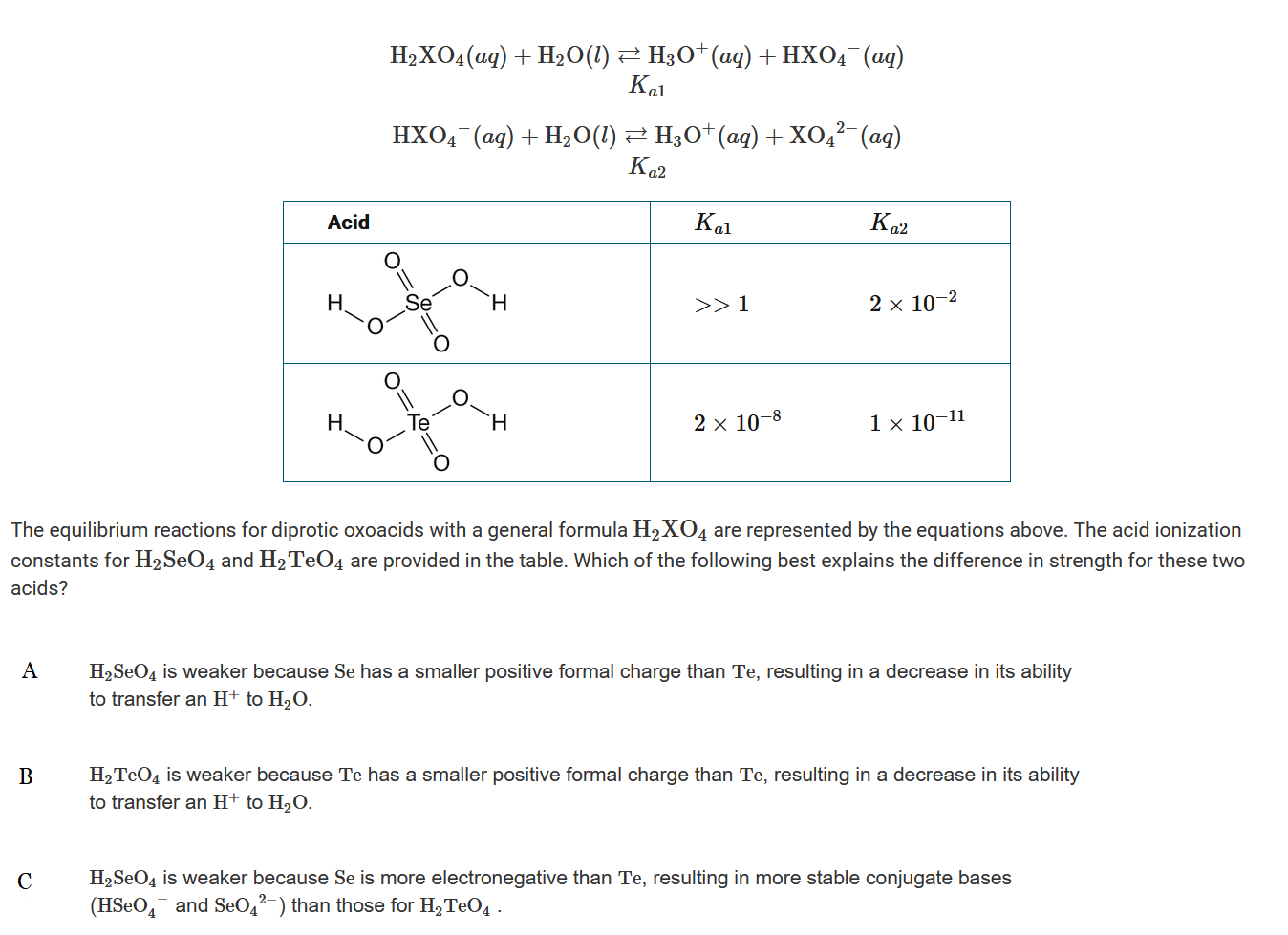
![]()
▶️Answer/Explanation
Ans:D
The chemical structure of these acids only show a difference in the central atom. The conjugate bases \(HTeO_4^−\) and \(TeO_4^{2−}\) are less stable than the conjugate bases \(HSeO_4^−\) and \(SeO_4^{2−}\) because Te is less electronegative then Se. The lower stability of the conjugate base results in lower values for \(K_{a1}\) and \(K_{a2}\), which indicates that \(H_2TeO_4\) is the weaker acid.
Question

Lewis diagrams of the weak bases \(NH_3\) and \(NF_3\) are shown above. Based on these diagrams, which of the following predictions of their relative base strength is correct, and why?
A \(NF_3\) is a stronger base than \(NH_3\) because more than one resonance structure exists for its conjugate acid NF3H+, making it more stable than \(NH^{4+}\).
B \(NF_3\) is a stronger base than \(NH_3\) because of the greater electronegativity of F compared with H.
C \(NF_3\) is a weaker base than \(NH_3\) because of the greater electronegativity of F compared with H.
D \(NF_3\) is a weaker base than \(NH_3\)because more than one resonance structure exists for its conjugate acid \(NF_3H^+\), making it more stable than \(NH_4^+\).
▶️Answer/Explanation
Ans:C
\(NF_3\) is a weaker base because the large electronegativity of the F atom results in \(NF_3\) being a poorer acceptor of protons than \(NH_3\) is.
Question
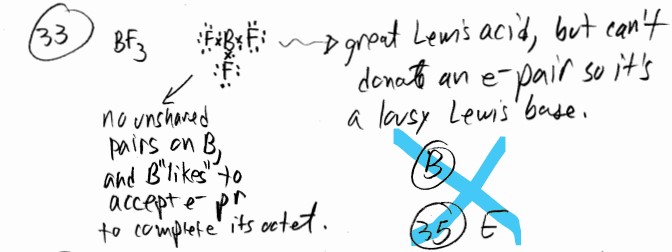
Which one of the following cannot act as a Lewis base?
A) \(NH_3\) B) \(BF_3\) C) \(Cl^-\) D) \(CN^-\) E) \(H_2O\)
▶️Answer/Explanation
Ans: B