Question
A student places a mixture of plastic beads consisting of polypropylene (PP) and polyvinyl chloride (PVC) in a 1.0 L beaker containing distilled water. After stirring the contents of the beaker vigorously, the student observes that the beads of one type of plastic sink to the bottom of the beaker and the beads of the other type of plastic float on the water. The chemical structures of PP and PVC are represented by the diagrams below, which show segments of each polymer.
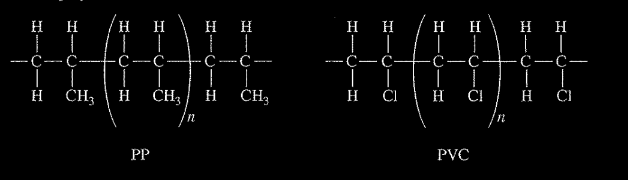
(a) Given that the spacing between polymer chains in PP and PVC is similar, the beads that sink are made of which polymer? Explain. PP is synthesized from propene, \(C_3H_6\), and PVC is synthesized from vinyl chloride, \(C_2H_2CI\). The structures of the molecules are shown below.
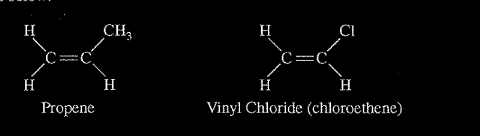
(b) The boiling point of liquid propene (226 K) is lower than the boiling point of liquid vinyl chloride (260 K). Account for this difference in terms of the types and strengths of intermolecular forces present in each liquid.
In a separate experiment, the student measures the enthalpies of combustion of propene and vinyl chloride. The student determines that the combustion of 2.00 mol of vinyl chloride releases 2300 kJ of energy, according to the equation below.
\(2 C_2H _3Cl(g) + 5 O_2(g) → 4 CO_2(g) + 2 H_2O(g) + 2 HCl\)(g) \( \Delta H^0\)=-2300 kJ/mol
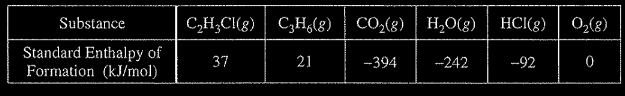
(c) Using the table of standard enthalpies of formation below, determine whether the combustion of 2.00 mol of propene releases more, less, or the same amount of energy that 2.00 mol of vinyl chloride releases. Justify your answer with a calculation. The balanced equation for the combustion of 2.00 mol of propene is
\(2 C_2H(g) + 9 O_2(g) → 6 CO_2(g) + 6 H_2O\)(g).
▶️Answer/Explanation
(a) The PVC beads sink. The spacing between chains is similar, but a Cl atom has a greater mass than \(CH_3\).
(b) Both substances have dipole-dipole interactions and London dispersion forces (or propene is essentially nonpolar with only LDFs while vinyl chloride has both LDFs and dipole-dipole forces). Propene contains a CH, group, but vinyl chloride contains a Cl atom. Vinyl chloride thus has a larger electron cloud, is more polarizable, and has a larger dipole moment. Thus intermolecular attractions are stronger in vinyl chloride, which results in it having the higher boiling point.
(c) ∆H°\(=6(-394) + 6(-242)-2(21)=-3858 kJ/mol_{rxn}\) The combustion of 2.00 mol of propene releases more energy.
Question
A student investigates the enthalpy of solution, ΔHsoln , for two alkali metal halides, LiCl and NaCl. In addition to the salts, the student has access to a calorimeter, a balance with a precision of ±0.1 g, and a thermometer with a precision of ±0.1°C.
(a) To measure ΔHsoln for LiCl, the student adds 100.0 g of water initially at 15.0°C to a calorimeter and adds 10.0 g of LiCl(s), stirring to dissolve. After the LiCl dissolves completely, the maximum temperature reached by the solution is 35.6°C.
(i) Calculate the magnitude of the heat absorbed by the solution during the dissolution process, assuming that the specific heat capacity of the solution is 4.18 J/(g·°C). Include units with your answer.
(ii) Determine the value of ΔHsoln for LiCl in kJ/molrxn .
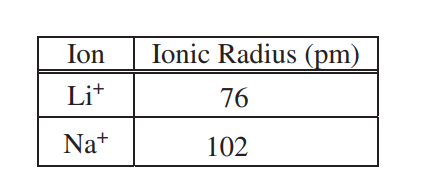
To explain why ΔHsoln for NaCl is different than that for LiCl, the student investigates factors that affect ΔHsoln and finds that ionic radius and lattice enthalpy (which can be defined as the ΔH associated with the separation of a solid crystal into gaseous ions) contribute to the process. The student consults references and collects the data shown in the table below.
(b) Write the complete electron configuration for the Na+ ion in the ground state.
(c) Using principles of atomic structure, explain why the Na+ ion is larger than the Li+ ion.
(d) Which salt, LiCl or NaCl, has the greater lattice enthalpy? Justify your answer.
(e) Below is a representation of a portion of a crystal of LiCl. Identify the ions in the representation by writing the appropriate formulas ( Li + or Cl− ) in the boxes below.
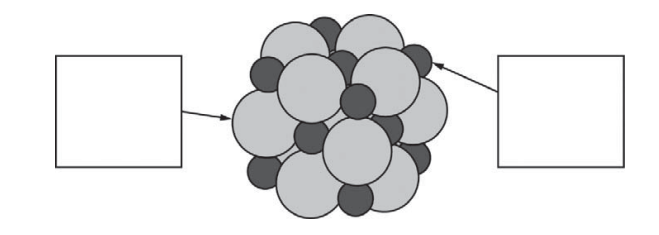
(f) The lattice enthalpy of LiCl is positive, indicating that it takes energy to break the ions apart in LiCl.
However, the dissolution of LiCl in water is an exothermic process. Identify all particle-particle interactions that contribute significantly to the dissolution process being exothermic. For each interaction, include the particles that interact and the specific type of intermolecular force between those particles.
▶️Answer/Explanation
Ans:
(a) (i)
| q = mcΔT = (110.0 g)(4.18 J/(g·°C))(35.6°C – 15.0°C) = 9,470 J = 9.47 kJ |
(ii)
\(10.0 g LiCl\times \frac{1 mol LiCl}{42.39 g LiCl}=0.236 mol LiCl\) \(\frac{-9.47 kJ}{0.236 mol LiCl}=-40.1 kJ/mol_{rxn}\) |
(b)
| 1s2 2s2 2p6 |
(c)
| The valence electrons in the Na+ ion are in a higher principal energy level than the valence electrons in the Li+ ion. Electrons in higher principal energy levels are, on average, farther from the nucleus. |
(d)
| LiCl. Because the Li+ ion is smaller than the Na+ ion, the Coulombic attractions between ions in LiCl are stronger than in NaCl. This results in a greater lattice enthalpy. |
(e)
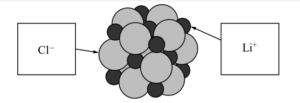
| See diagram above. |
(f)
| There are interactions between Li+ ions and polar water molecules and between Cl– ions and polar water molecules. These are ion-dipole interactions. |
Question
NaHCO3(s) + HC2H3O2(aq) → NaC2H3O2(aq) + H2O(l) + CO2(g)
A student designs an experiment to study the reaction between NaHCO3 and HC2H3O2 . The reaction is represented by the equation above. The student places 2.24 g of NaHCO3 in a flask and adds 60.0 mL of 0.875 M HC2H3O2 . The student observes the formation of bubbles and that the flask gets cooler as the reaction proceeds.
(a) Identify the reaction represented above as an acid-base reaction, precipitation reaction, or redox reaction. Justify your answer.
(b) Based on the information above, identify the limiting reactant. Justify your answer with calculations.
(c) The student observes that the bubbling is rapid at the beginning of the reaction and gradually slows as the reaction continues. Explain this change in the reaction rate in terms of the collisions between reactant particles.
(d) In thermodynamic terms, a reaction can be driven by enthalpy, entropy, or both.
(i) Considering that the flask gets cooler as the reaction proceeds, what drives the chemical reaction between NaHCO3(s) and HC2H3O2(aq) ? Answer by drawing a circle around one of the choices below.
Enthalpy only Entropy only Both enthalpy and entropy
(ii) Justify your selection in part (d)(i) in terms of ΔG°.
(e) The HCO3− ion has three carbon-to-oxygen bonds. Two of the carbon-to-oxygen bonds have the same length and the third carbon-to-oxygen bond is longer than the other two. The hydrogen atom is bonded to one of the oxygen atoms. In the box below, draw a Lewis electron-dot diagram (or diagrams) for the HCO3− ion that is (are) consistent with the given information.

(f) A student prepares a solution containing equimolar amounts of HC2H3O2 and NaC2H3O2 . The pH of the solution is measured to be 4.7. The student adds two drops of 3.0 M HNO3(aq) and stirs the sample, observing that the pH remains at 4.7. Write a balanced, net-ionic equation for the reaction between HNO3(aq) and the chemical species in the sample that is responsible for the pH remaining at 4.7.
▶️Answer/Explanation
Ans:
(a)
| It is an acid-base reaction. The weak acid HC2H3O2 reacts with the weak base HCO3– with HC2H3O2 donating a proton. OR It is an acid-base reaction. No solid precipitates, so it is not a precipitation reaction. None of the oxidation numbers change, so it is not a redox reaction. |
(b)
\(2.24 g NaHCO_{3}\times \frac{1 mol NaHCO_{3}}{84.0 g}= 0.0267 mol NaHCO_{3}\) \(60.0 mL\times \frac{0.875 mol HC_{2}H_{3}O_{2}}{1000 mL}= 0.0525 mol HC_{2}H_{3}O_{2}\) The NaHCO3(s) and HC2H3O2 (aq) react in a 1:1 ratio, so the limiting reactant is NaHCO3(s). |
(c)
| As the reaction proceeds, both reactants are consumed and their concentrations decrease. Collisions between reactant particles become less likely as their concentrations decrease, thus the reaction rate slows. |
(d) (i)
| Entropy only should be circled. |
(ii)
ΔG0 = ΔH0 – TΔS0 Reactions are thermodynamically favorable when ΔG0 is negative. Since the reaction is endothermic (the flask cooler, ΔH0 is positive), the reaction is not driven by enthalpy, because enthalpy does not help make ΔG0 negative. Because there are no gases in the reactants and one of the products is a gas, ΔS0 must be positive, which helps make ΔG0 negative. |
(e)

| See diagram above. |
(f)
| C2H3O2– + H3O+ → HC2H3O2 + H2O OR C2H3O2– + H+ → HC2H3O2 |
