Question
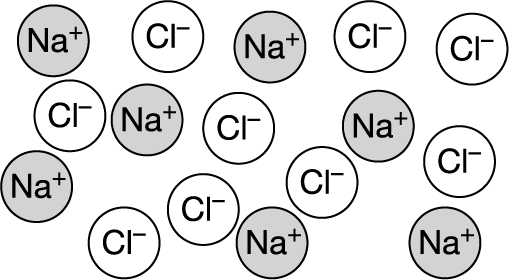
Molten (liquid) NaCl is represented by the particulate diagram shown above. Which of the following indicates whether NaCl(l) conducts electricity and best explains why or why not?
A It conducts electricity because Na is a metal.
B It conducts electricity because ions are free to move.
C It does not conduct electricity because Cl is a nonmetal.
▶️Answer/Explanation
Ans: B For a substance to conduct electricity, it must contain charged particles that are mobile. In molten salts such as NaCl , those charged particles are ions. (Metals conduct electricity via mobile electrons within a lattice of metal atoms.)
Question
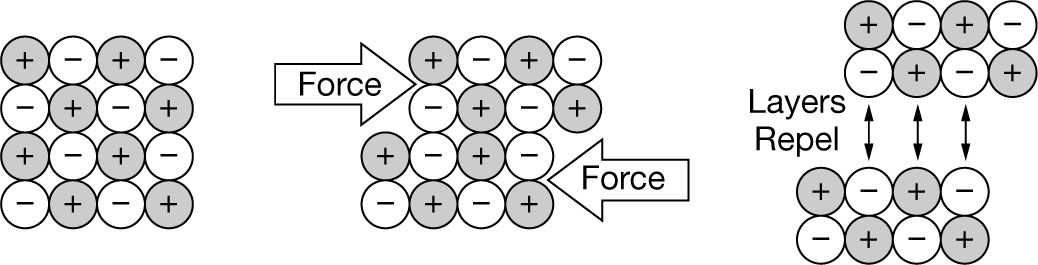
The particulate-level diagram shown above best helps to explain which of the following properties of ionic solids?
A Density
B Brittleness
C Malleability
▶️Answer/Explanation
Ans: B The brittleness of ionic solids is due to the internal repulsion of ion layers within the crystal lattice that occurs when the layers are displaced by an outside force parallel to the layers and they move relative to adjacent layers.
Question
The particles in solid KI, a stable ionic compound, are arranged to maximize coulombic attractions while minimizing coulombic repulsions among the particles. Which of the following diagrams best represents the structure of solid KI?
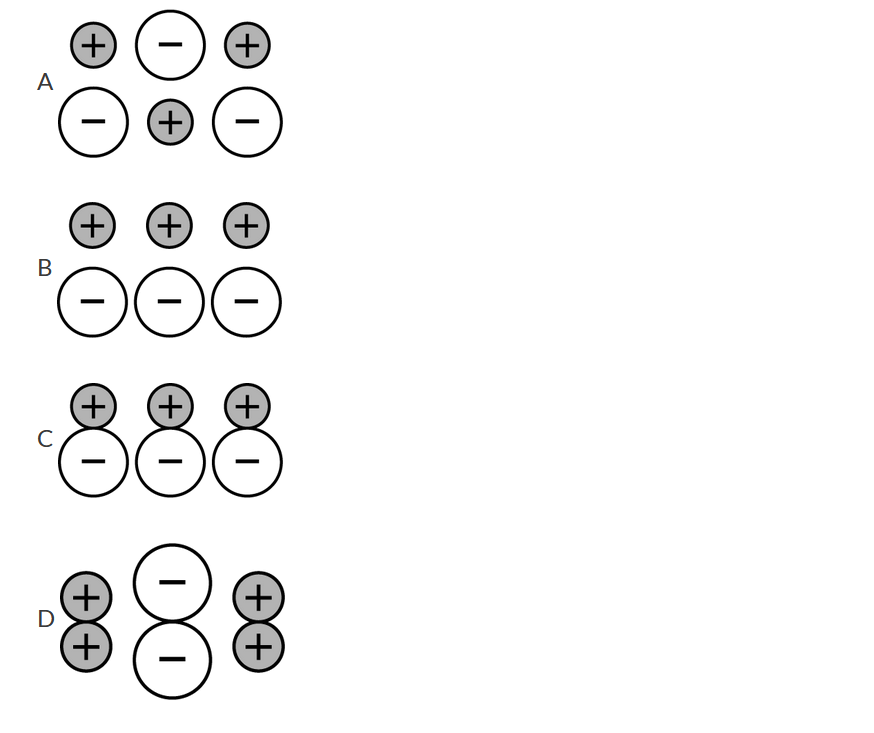
▶️Answer/Explanation
Ans:A This diagram shows both types of charged ions being surrounded by ions of the opposite charge, thus maximizing attraction and minimizing repulsion.
Question
Steel is an alloy consisting of Fe with a small amount of C. Elemental Cr can be added to steel to make the steel less likely to rust; Cr atoms react with oxygen in the air to form a nonreactive layer of chromium oxide on the surface of the steel, preventing the oxidation of underlying Fe atoms. A sample of steel-chromium alloy contains 15 percent Cr by mass. Which of the following diagrams best shows a particle-level view of a surface section and an interior section of the alloy represented below at the left? (The atomic radii of the atoms involved are given in the table below at the right)
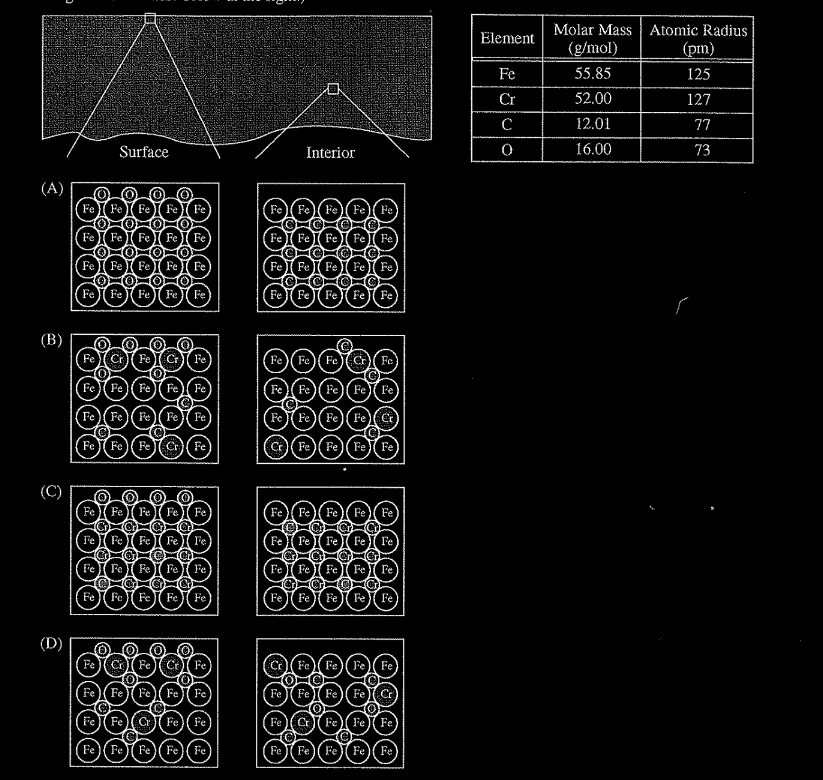
▶️Answer/Explanation
Ans:B
Question
Which of the following diagrams best illustrates how a displacement in an ionic crystal results in cleavage and brittleness?
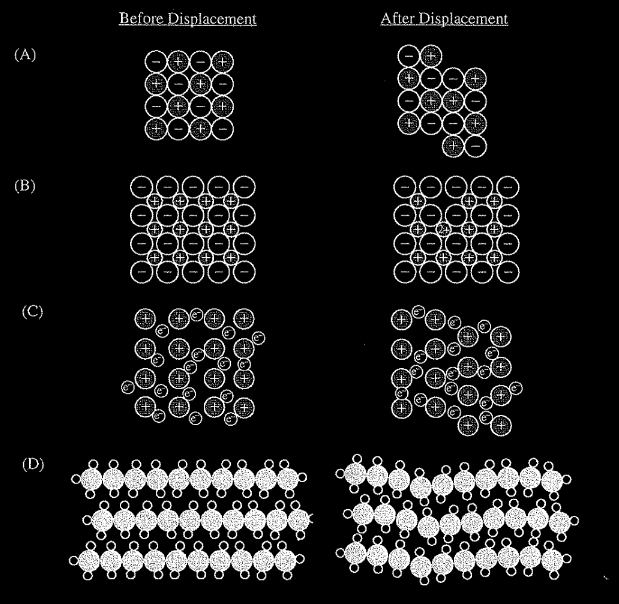
▶️Answer/Explanation
Ans:A
