IB Biology SL (Standard level)- 2024 – Practice Questions- All Topics
Topic 2.1 Molecules to metabolism
Topic 2 Weightage : 20%
All Questions for Topic 2.1 –Molecular Biology, Organic Compounds, Organic Subunits -glucose, ribose and fatty and amino acids, Organic Polymers -sugars and lipids, Falsifying Vitalism, Metabolism, Anabolism and Catabolism, Periodic Table, Types of Bonding, Trace Elements
Question
Which molecule is depicted in the diagram?
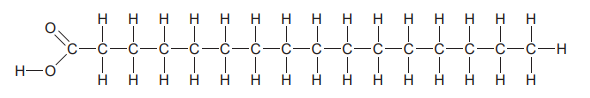
A saturated fatty acid
An unsaturated fatty acid
A trans fat
A vegetable oil
Answer/Explanation
Ans: A
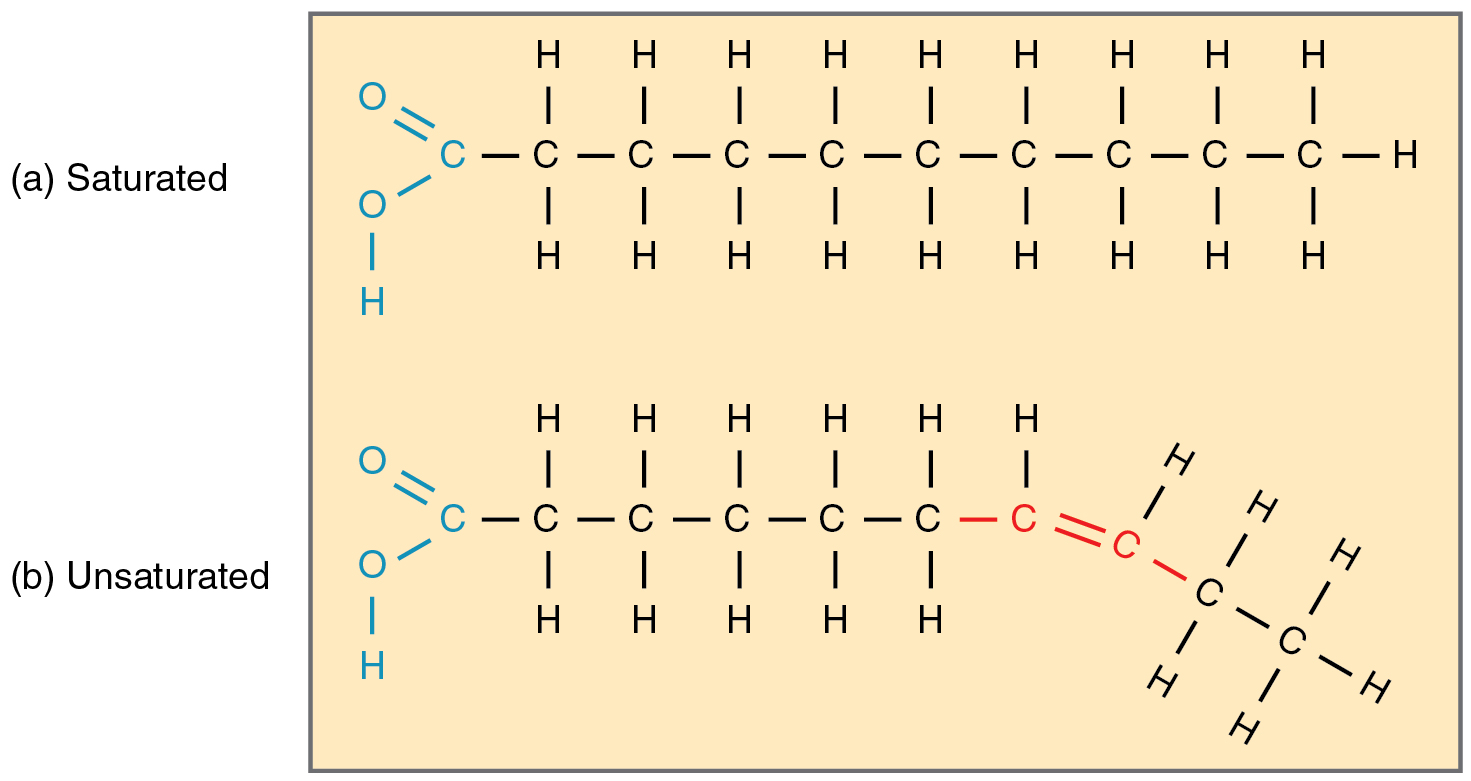
Saturated fatty acids are a type of fatty acid that have a perfectly straight chain structure. In biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated.
The key difference between saturated and unsaturated fats is that saturated fats do not have double bonds between fatty acid chains while unsaturated fats have double bonds in the fatty acid chains. Saturated fats are generally solid at room temperature and don’t get spoiled easily. Whereas unsaturated fats are liquids at room temperature, and they spoil quickly.
Question
What distinguishes cellulose from glycogen and starch?
Only cellulose is found in plants.
Only cellulose is made up of glucose monomers.
Cellulose is far more branched than starch and glycogen.
Cellulose has a structural role whereas starch and glycogen function in energy storage.
Answer/Explanation
Ans: D
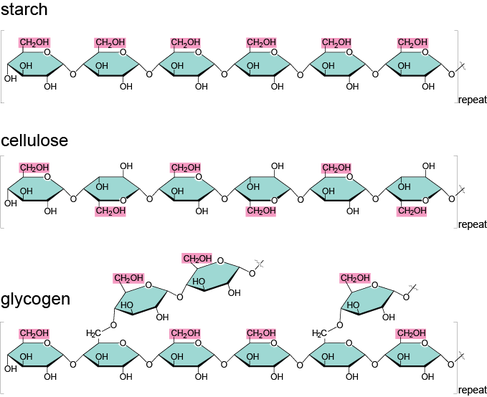
Glycogen and starch are both polymers of glucose, but they differ in their structure and biological sources. Glycogen is produced by animals and is known as animal starch, while starch is produced by plants. Glycogen has a branched structure, while starch has both chain and branched components. Glycogen and starch are both complex carbohydrates or polysaccharides. They serve as energy storage molecules, but they differ in their biological sources, structure, and the organisms that utilize them for energy.
Starch is a complex carbohydrate made by plants to store excess glucose that is not used right away for cellular work. Starch has a fairly rigid structure and can be molded into different shapes. Plants use the stored starch to survive when sunlight is not available for photosynthesis. Some starchy foods are rice, corn, bread, potatoes, tapioca, millet, and pasta.
Glycogen is a complex carbohydrate made by animals to store excess glucose that is not used right away for cellular work. Glycogen has a highly branched structure and is mainly stored in the liver and muscles. Glycogen helps cells store glucose by providing them with a constant supply of energy. Glycogen also plays an important role in the glucose cycle.
The diagram shows the structure of palmitic acid.
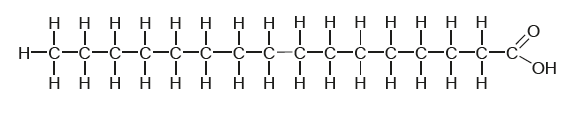
What type of fatty acid is palmitic acid?
A. It is monounsaturated.
B. It is polyunsaturated.
C. It is saturated.
D. It is a trans-fatty acid.
Answer/Explanation
Markscheme
C
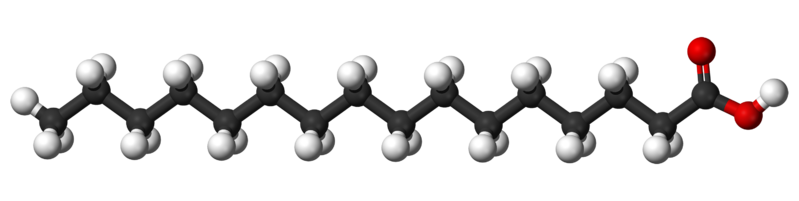
Palmitic acid is a saturated long-chain fatty acid containing 16 carbons. It is the most common saturated fatty acid found in animals, plants and microorganisms. It is found in most fats and oils such as soybean oil.
The diagram shows a cycle of reactions that occurs in human liver cells.
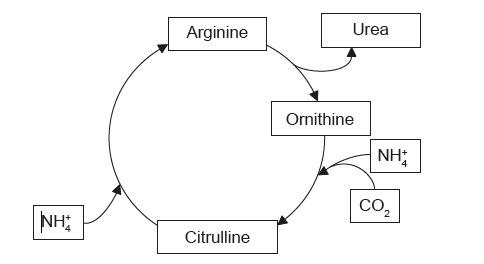
Which term describes the overall reactions of this cycle?
A. Oxidation
B. Catabolism
C. Condensation
D. Metabolism
Answer/Explanation
Markscheme
D
The overall reaction of metabolism that occurs in human liver cell is complex and involves many pathways and enzymes. However, a simplified summary can be given as follows:
– The liver is an essential metabolic organ that regulates carbohydrate, lipid, protein and energy metabolism.
– Glucose is metabolized into pyruvate through glycolysis in the cytoplasm, and pyruvate is completely oxidized to generate ATP through the TCA cycle and oxidative phosphorylation in the mitochondria.
– Excess glucose is converted into glycogen and stored in the liver cells or released into the bloodstream as needed.
– Fatty acids are synthesized from acetyl-CoA or taken up from the blood and stored as triglycerides in the liver cells or exported as lipoproteins to other tissues.
– The liver also produces bile, an alkaline fluid that helps the breakdown of fat in the small intestine.
– Amino acids are deaminated and transaminated in the liver, followed by conversion of the non-nitrogenous part of those molecules to glucose or lipids.
– The liver also detoxifies drugs and toxins, stores and modifies vitamins and iron, and synthesizes plasma proteins and hormones
Which molecule diagram corresponds to the name?
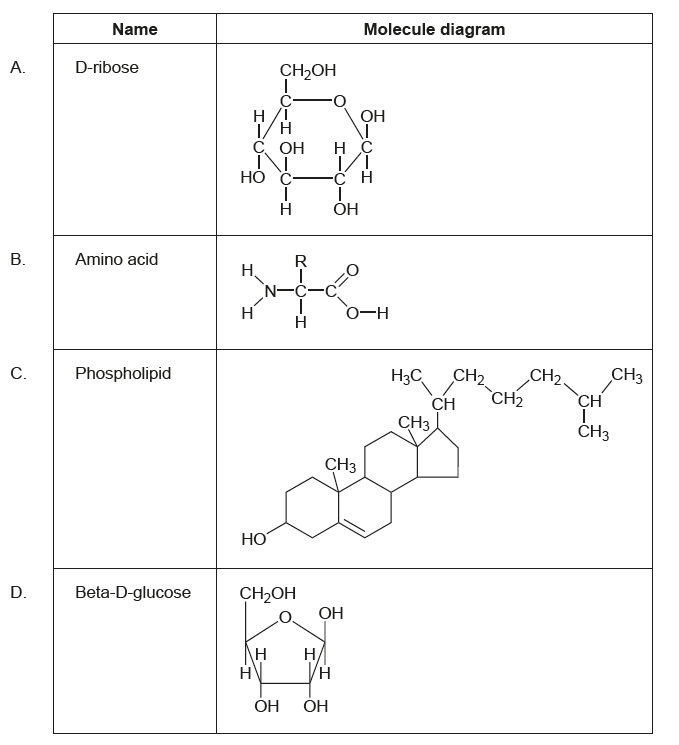
Answer/Explanation
Markscheme
B
Question
Which process is an example of catabolism?
Translation of mRNA
Replication of DNA
Hydrolysis of protein
Synthesis of a disaccharide
▶️Answer/Explanation
Ans: C
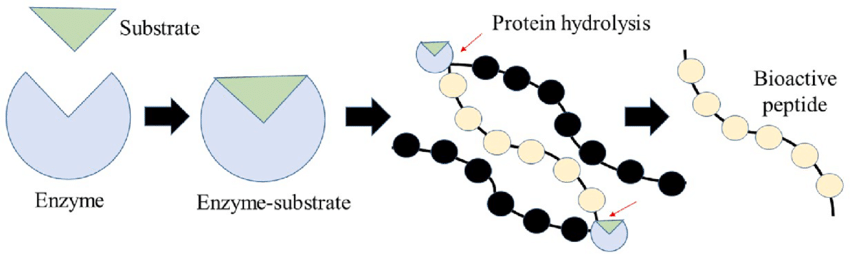
Hydrolysis is a catabolic reaction because it breaks down large molecules into smaller ones. In the case of proteins, hydrolysis breaks down the peptide bonds between amino acids,
which are the building blocks of proteins. This process is catalyzed by enzymes called hydrolases.
Question
The diagram shows the product of a polymerization reaction.
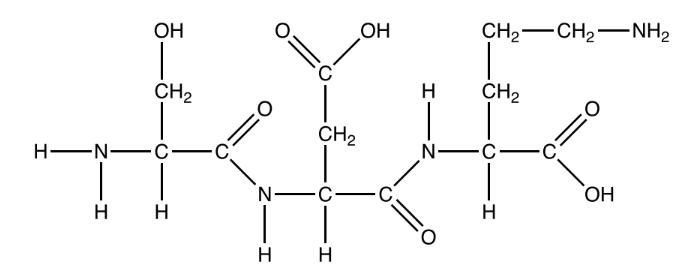
What is formed in this polymerization reaction?
A. A dipeptide formed by the hydrolysis of two nucleotides
B. A tripeptide formed by the hydrolysis of three amino acids
C. A dipeptide formed by the condensation of two amino acids
D. A tripeptide formed by the condensation of three amino acids
▶️Answer/Explanation
Ans:C
Question
The diagram shows water molecules.
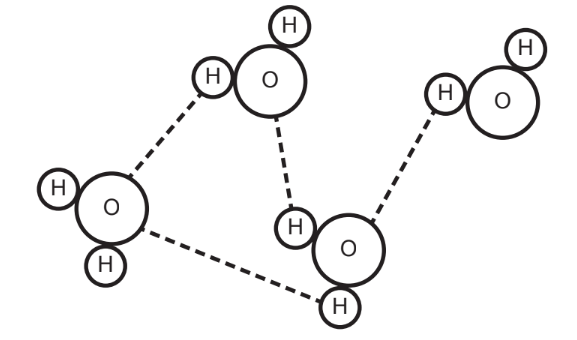
Which property of water is not illustrated?
A. Cohesion
B. Dipolarity
C. Hydrogen bonding
D. Adhesion
▶️Answer/Explanation
Ans:C
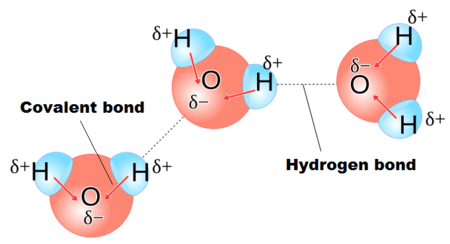
A water molecule is said to be dipolar because it has a positive and a negative pole as a result of the uneven distribution of electrons within it. The dipole nature within a water molecule creates attractive forces known as hydrogen bonding, allowing them to stick together. The oxygen atom in the water molecule is attached to two hydrogen atoms.