IB PHYSICS SL (Standard level)- 2024 – Practice Questions- All Topics
Topic 3.2 – Modelling a gas
Topic 3 Weightage : 6 %
All Questions for Topic 3.2 – Pressure , Equation of state for an ideal gas , Kinetic model of an ideal gas . Mole, molar mass and the Avogadro constant , Differences between real and ideal gases
Question
A quantity of 2.00 mol of an ideal gas is maintained at a temperature of 127 0C in a container of volume 0.083 m3. What is the pressure of the gas?
A 8 kPa
B 25 kPa
C 40 kPa
D 80 kPa
▶️Answer/Explanation
Ans: D
Ref:https://www.iitianacademy.com/ib-physics-unit-3-modelling-a-gas-notes/
We have Ideal Gas law as
ideal gas law:
An ideal gas being a gas that obeys the ideal gas law at all pressures, volumes and temperatures. No real gas is ideal, but most gases obey the ideal gas law for low pressure and high temperatures (above their freezing point).
R is called the universal gas constant, the value of R is dependent on the choice of units:
Question
In the kinetic model of an ideal gas, which of the following is not assumed?
A. The molecules collide elastically.
B. The kinetic energy of a given molecule is constant.
C. The time taken for a molecular collision is much less than the time between collisions.
D. The intermolecular potential energy of the molecules is zero.
▶️Answer/Explanation
Markscheme
B
Ref:https://www.iitianacademy.com/ib-physics-unit-3-modelling-a-gas-notes/
- A gas consist of particles called molecules which move randomly in all directions.
- The volume of molecule is very small in comparison to the volume occupied by gas i.e., the size of molecule is infinitesimally small.
- The collision between two molecules or between a molecule and wall are perfectly elastic and collision time (duration of collision) is very small.
- The molecules exert no force on each other or on the walls of containers except during collision.
- The total number of molecules are large and they obey Newtonian mechanics.
The internal energy of a perfect gas consists only of kinetic energy of the molecules. But in case of the real gas it consists of both the kinetic energy and potential energy of inter molecular configuration.
Question
What is the definition of the mole?
A. The amount of substance that has the same mass as 6.02 × 1023 atoms of carbon-12
B. The amount of substance that contains as many nuclei as the number of nuclei in 12 g of carbon-12.
C. The amount of substance that has the same mass as one atom of carbon-12.
D. The amount of substance that contains as many elementary entities as the number of atoms in 12 g of carbon-12.
▶️Answer/Explanation
Markscheme
D
A mole (mol) is the number of particles in a sample such that the mass in grams of the sample is equal to the mass in amu of a single particle. So 1 mol of carbon-12 atoms has a mass of 12 g, 1 mol of helium-4 has a mass of 4 g.
Question
Which of the following is an assumption of the kinetic model of an ideal gas?
A. The gas is at high pressure.
B. There are weak forces of attraction between the particles in the gas.
C. The collisions between the particles are elastic.
D. The energy of the particles is proportional to the absolute temperature.
▶️Answer/Explanation
Markscheme
C
- A gas consist of particles called molecules which move randomly in all directions.
- The volume of molecule is very small in comparison to the volume occupied by gas i.e., the size of molecule is infinitesimally small.
- The collision between two molecules or between a molecule and wall are perfectly elastic and collision time (duration of collision) is very small.
- The molecules exert no force on each other or on the walls of containers except during collision.
- The total number of molecules are large and they obey Newtonian mechanics.
Question
An ideal gas is contained in a thermally insulated cylinder by a freely moving piston.
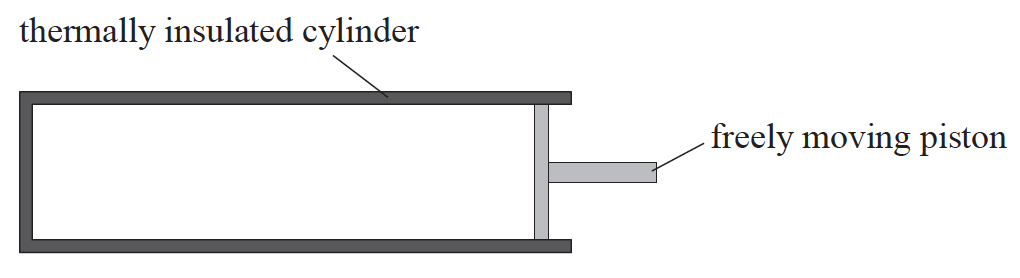
The gas is compressed by the piston and as a result the temperature of the gas increases. What is the explanation for the temperature rise?
A. The rate of collision between the molecules increases.
B. Energy is transferred to the molecules by the moving piston.
C. The molecules of the gas are pushed closer together.
D. The rate of collision between the molecules and the walls of the cylinder increases.
▶️Answer/Explanation
Markscheme
B
Suppose the piston is pushed slowly to compress the gas. As a gas molecule collides with the piston coming towards it, the speed of the molecule increases on
collision (assuming elastic collision, \(v_2=v_1+2u\) in figure ). This way the internal energy of the molecules increases as the piston is pushed in.
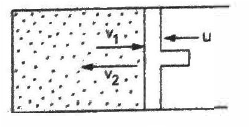
Examiners report
The weaker candidates were opting for D. But D does not answer the question which asks for an explanation for the temperature rise.
Question
The energy of the molecules of an ideal gas is
thermal only.
thermal and potential.
potential and kinetic.
kinetic only.
▶️Answer/Explanation
Markscheme
The internal energy of a perfect gas consists only of kinetic energy of the molecules. But in case of the real gas it consists of both the kinetic energy and potential energy of inter molecular configuration.