IB PHYSICS SL (Standard level)- 2024 – Practice Questions- All Topics
Topic 1.1 Measurement in Physics
Topic 1 Weightage : 9 %
All Questions for Topic 1.1 – SI units, calculations and presentation of raw and processed data, scientific notation and metric multipliers, Quoting and comparing ratios, values and approximations to the nearest order of magnitude. Significant figures, Orders of magnitude, Estimation
Question
What is the order of magnitude of the wavelength of visible light?
A. 10-10 m
B. 10-7 m
C. 10-4 m
D. 10-1 m
Answer/Explanation
Ans
#
Visible light which is detectable by the human eye consists of wavelength ranging from approximately . So, the wavelength of light visible to the eye is of the order of
Question
Which quantity has the same units as those for energy stored per unit volume?
A Density
B Force
C Momentum
D Pressure
Answer/Explanation
Ans: D
\(\frac{Energy}{Volume}=\frac{work}{area\times length}=\frac{force \times distance}{area\times length}\)
\(=\frac{MLT^{-2}\times L}{L^2\times L}=\left [ ML^{-1}T^{-2} \right ]=pressure\)
Question
Which is a unit of force?
A. J m
B. J m–1
C. J m s–1
D. J m–1 s
Answer/Explanation
Markscheme
B
A joule per meter (J/m) is a metric unit of force. In mechanics, a joule is defined as the work done by a force of one newton acting to move an object through a distance of one meter in the direction in which the force is applied (1 J = 1 N·m). Therefore, joule per meter is equal to newton.
Question
What is the best estimate for the diameter of a helium nucleus?
A. 10–21 m
B. 10–18 m
C. 10–15 m
D. 10–10 m
Answer/Explanation
Markscheme
C
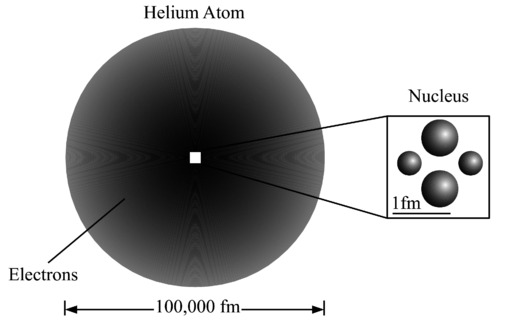
The size of the helium nucleus is about 1 fermi, or 1 fm, which is equivalent to 10-15 m. The atom is about 100,000 times bigger than the nucleus, with an atom size of about 105 fm or 10-10 m.
Question
How many significant figures are there in the number 0.0450?
A. 2
B. 3
C. 4
D. 5
Answer/Explanation
Markscheme
B
The first one being that all non-zero numbers are significant. With a 4 and a 5 in the number, we know there are at least 2 significant digits.
Lastly , is that a trailing zero after a decimal is significant. So the 450 is significant. The zeros in front of the decimal are irrelevant.
Therefore, there are 3 significant digits in this number. Please visit below link for more details
IB Physics Unit 1. Measurements and uncertainties- 1.1 Measurements in physics: Study Notes