Question
Which substance is likely to have an ionic lattice structure at 298K and 100kPa?

▶️Answer/Explanation
Markscheme : D
The characteristics that suggest an ionic lattice structure are a high melting point and the ability to conduct electricity in a molten state (liquid) or when dissolved in water. Ionic compounds generally have high melting points due to the strong electrostatic forces holding the ions together in a lattice structure.
So, based on the provided information, the substance likely to have an ionic lattice structure is the one with a high melting point and the ability to conduct electricity in a liquid state.
Therefore, the correct choice is:
High melting point & Conducts electricity in a liquid state? – This combination suggests an ionic lattice structure.
So, the correct answer is “high & yes”.
Question
What are the correct formulas of the following ions?
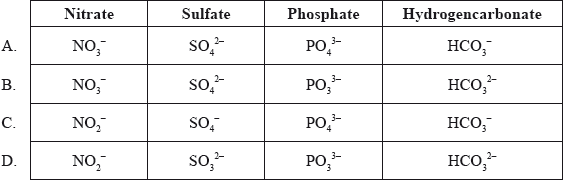
Answer/Explanation
Markscheme
A
Examiners report
Question
Which compounds have an ionic lattice structure in the solid state?
I. Silicon dioxide
II. Sodium fluoride
III. Ammonium nitrate
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answer/Explanation
Markscheme
C
Examiners report
Question
The formula of gallium phosphate is \({\text{GaP}}{{\text{O}}_{\text{4}}}\). What is the correct formula of gallium sulfate?
A. \({\text{GaS}}{{\text{O}}_{\text{4}}}\)
B. GaS
C. \({\text{G}}{{\text{a}}_{\text{2}}}{{\text{(S}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{3}}}\)
D. \({\text{G}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{3}}}\)
Answer/Explanation
Markscheme
C
Examiners report
Question
A substance has the following properties:

What is the most probable structure of this substance?
A. Network covalent
B. Polar covalent molecule
C. Ionic lattice
D. Metallic lattice
Answer/Explanation
Markscheme
A