Question-1 :2020-nov-Chemistry_paper_1__TZ0_SL
Topic:
Calculate: What is the molar mass, in $\mathrm{g} ~\mathrm{mol}^{-1}$, of a compound if $0.200 \mathrm{~mol}$ of the compound has a mass of $13.2 \mathrm{~g}$ ?
A. $66.0$
B. $66$
C. $26.4$
D. $26$
Answer/Explanation
Solution:
$
\text { Molar mass }=\frac{\text { given mass }}{\text { moles }}
$
Molar mass of compound $=\frac{13.2 \mathrm{~g}}{0.2 \mathrm{~mol}}=66.0 \mathrm{~g} \mathrm{~mol}^{-1}$
Question-2 :2020-nov-Chemistry_paper_1__TZ0_SL
Topic:
Calculate: What is the number of carbon atoms in $12 \mathrm{~g}$ of ethanoic acid $\mathrm{CH}_3 \mathrm{COOH}, M_{\mathrm{r}}=60$ ?
A. $ 0.20$
B. $ 2.0$
C. $1.2 \times 10^{23}$
D. $ 2.4 \times 10^{23}$
Answer/Explanation
Solution:
Number of carbonatoms in 1 molecule of ethanoic acid $=2$
Number of moles of $\mathrm{C}$ in 1 mole of ethanoic acid $=2$ moles
Number of moles of ethanoic acid $=\frac{12}{60}=\frac{1}{5}$
Number of moles of carbon $=2 \times \frac{1}{5}=\frac{2}{5}$
Number of carbonatoms in 1 mole $=6.023 \times 10^{23}$
Number of carbon atoms in $2 / 5$ moles $=\frac{2}{5} \times 6.023 \times 10^{23}=2.4 \times 10^{23}$ atoms
Question-3 :2020-nov-Chemistry_paper_1__TZ0_SL
Topic:
Discuss: Which of these molecular formulae are also empirical formulae?
- $\mathrm{C}_2 \mathrm{H}_6 \mathrm{O}$
- $\mathrm{C}_2 \mathrm{H}_4 \mathrm{O}_2$
- $\mathrm{C}_5 \mathrm{H}_{12}$
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answer/Explanation
Solution:
Empirical formula of a compound is the formula which represents the simplest ratio of the elements
I $\mathrm{C}_2 \mathrm{H}_6 \mathrm{O}$ ratio is $2: 6: 1$ which is simplest
II $\mathrm{C}_2 \mathrm{H}_4 \mathrm{O}_2$ ratio is $2: 4: 2$ which is not simplest can be changed $1: 2: 1$
III $\mathrm{C}_5 \mathrm{H}_{12}$ ratio is $5: 12$ which is simplest
So I and III are empinical formula
Question-4 :2020-nov-Chemistry_paper_1__TZ0_SL
Topic:
Calculate: Which volume of ethane gas, in $\mathrm{cm}^3$, will produce $40 \mathrm{~cm}^3$ of carbon dioxide gas when mixed with $140 \mathrm{~cm}^3$ of oxygen gas, assuming the reaction goes to completion?
$$
2 \mathrm{C}_2 \mathrm{H}_6(\mathrm{~g})+7 \mathrm{O}_2(\mathrm{~g}) \rightarrow 4 \mathrm{CO}_2(\mathrm{~g})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{g})
$$
A. $10$
B. $20$
C. $40$
D. $80$
Answer/Explanation
Solution:
We assume that volume $\approx$ moless. so we have 40 moles of carbon dioxide and 140 moles of oxygen
According to balanced equation 7 moles of $\mathrm{O}_2$ will produce $=4$ moles of $\mathrm{CO}_2$
1 mole of $\mathrm{O}_2$ will produce $=\stackrel{4}{7}$ moles of $\mathrm{CO}_2$
140 moles of $\mathrm{O}_2$ will produce $=\frac{4}{7} \times 140=80$ moles of $\mathrm{CO}_2$
So carbon dioxide is limiting reagent and oxygen is in excess
According to balancedequation
4 moles of $\mathrm{CO}_2$ require $=2$ moles of $\mathrm{C}_2 \mathrm{H}_6$
1 mole of $\mathrm{CO}_2$ require $=\frac{2}{4}=\frac{1}{2}$ moles
40 moles require $=\frac{1}{2} \times 40=20$ moles of ethane
Question-5 :2020-nov-Chemistry_paper_1__TZ0_SL
Topic:
Calculate: What is the relative atomic mass, $A_r$ of an element with this mass spectrum?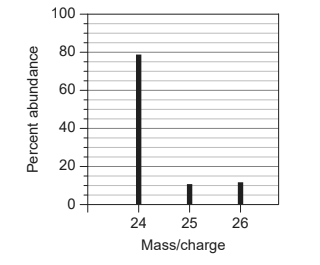
A. $24.0$
B. $24.3$
C. $24.9$
D. $25.0$
Answer/Explanation
Solution:
$\begin{aligned} & \text { Relative atomic mass }=\frac{\Sigma(\text { mass } \times \% \text { abundance })}{100} \\ & \text { Relative atomic mass } \mathrm{A}_{\mathrm{r}}=\frac{(24 \times 80)+(25 \times 10)+(26 \times 10)}{100} \\ & A_r=24.3 \mathrm{~g}\end{aligned}$
