Question
Which properties of water reduce temperature changes inside cells?
1 cohesion
2 latent heat of vaporisation
3 specific heat capacity
A 1 and 2 B 1 and 3 C 2 and 3 D 3 onl
▶️Answer/Explanation
Answer: D
The correct answer to the question “Which properties of water reduce temperature changes inside cells?” is B) 1 and 3, which means that both cohesion and specific heat capacity reduce temperature changes inside cells.
Cohesion is the property of water that allows water molecules to stick together due to hydrogen bonding. This property helps to maintain the integrity of water columns in plants and reduces temperature changes inside cells by allowing water to absorb heat without evaporating. This property helps to prevent water from evaporating rapidly and keeps the cells hydrated.
Specific heat capacity is the amount of heat energy required to raise the temperature of a substance by a given amount. Water has a high specific heat capacity, which means that it can absorb a large amount of heat energy without a significant increase in temperature. This property helps to regulate the temperature inside cells by buffering against temperature changes.
Therefore, both properties of cohesion and specific heat capacity are essential in reducing temperature changes inside cells. The correct answer is not D because latent heat of vaporization is not directly related to reducing temperature changes inside cells.
Question
Hydrogen bonding explains many of the properties of water, including the high latent heat of vapourisation and high specific heat capacity.
For which processes in plants is hydrogen bonding in water important on hot sunny days?
- preventing denaturation of enzymes in leaves
- reducing water loss by evaporation
- allowing leaves to cool down quickly at night
- holding the column of water in xylem vessels together
A. 1, 2, 3 and 4
B. 1, 2 and 4 only
C. 1, 3 and 4 only
D. 2 and 3 only
▶️Answer/Explanation
Ans:
B
On hot sunny days, hydrogen bonding in water is important for reducing water loss by evaporation (2) and holding the column of water in xylem vessels together (4).
Therefore, the answer is B) 1, 2, and 4 only.
Hydrogen bonding is the property of water that allows water molecules to stick together due to the attraction between the positive and negative ends of different molecules. This property helps to hold the column of water in xylem vessels together, allowing water to move up the plant against gravity. Additionally, hydrogen bonding helps to reduce water loss by evaporation by preventing water from evaporating rapidly.
While hydrogen bonding is important for maintaining the structure of enzymes (1) and allowing leaves to cool down quickly at night (3), these processes are not directly related to hot sunny days. Therefore, the correct answer is not A or C. Option D is incorrect because it only includes two out of the three correct answers.
Question
Polar molecules form hydrogen bonds with each other.
Which properties of water result from its molecules being polar?
1. good solvent
2. high specific heat capacity
3. high surface tension
4. cohesive
A. 1, 2, 3 and 4
B. 1, 2 and 3 only
C. 1, 2 and 4 only
D. 3 and 4 only
▶️Answer/Explanation
Ans:
A
A) 1, 2, 3, and 4, which means that water’s polarity results in it being a good solvent, having a high specific heat capacity, high surface tension, and cohesive.
Water’s polarity allows it to be a good solvent (1) for polar and ionic substances. The polarity of water also results in high specific heat capacity (2) because it can absorb a large amount of heat without a significant increase in temperature. Water’s polarity also results in high surface tension (3) due to the cohesive forces between water molecules, which form a “skin” on the surface of the water. Finally, the polarity of water also results in cohesion (4) between water molecules, which is the ability of water molecules to stick together due to hydrogen bonding.
Therefore, the correct answer is A) 1, 2, 3, and 4.
Question
When a lake begins to freeze, which properties of water are needed for fish to survive?
- Water has a high surface tension.
- Water has a high latent heat of vaporisation.
- Water has a high thermal capacity.
- Water has its maximum density at 4°C.
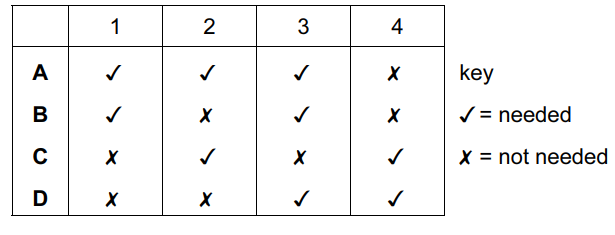
Answer/Explanation
Ans:
D
