Question
Enzymes are important biological molecules.
(a) Describe how an enzyme interacts with a substrate.
(b) Explain the difference between competitive inhibitors and
allosteric inhibitors of enzymes.
(c) An enzyme has its maximum efficiency at an optimum
temperature of 25° Celsius. Predict what effect a decrease in
temperature to 5° Celsius would have on the enzyme’s efficiency.
(d) Justify your prediction from part (c).
▶️Answer/Explanation
Ans:
(a) Enzymes interact with a specific substrate that has properties (such
as shape and charge) that are compatible with those at the enzyme’s
active site.
(b) Competitive inhibitors bind to enzymes at the active site, whereas
allosteric inhibitors bind to enzymes at the allosteric site.
(c) A decrease in temperature to 5° Celsius would decrease the
enzyme’s efficiency.
(d) Decreases in temperature reduce the number of molecular
collisions between the substrate and the enzyme, reducing the
number of chemical reactions and the enzyme’s efficiency.
Decreases in temperature may also alter the tertiary structure of the
enzyme, altering its active site and reducing its catalytic ability.
Question
Amylase is an enzyme that catalyzes the breakdown of amylose into its glucose subunits. The activity of amylase was measured at 37° Celsius
and a pH of 7, both of which are optimum conditions for the activity of this enzyme. The production of glucose was measured both with and
without the presence of compound X. Data are shown in the following table:
| Time (min) | Cumulative Amount of Glucose Produced (millimoles) in the Absence of Compound X | Cumulative Amount of Glucose Produced (millimoles) is the Presence of Compound X |
| 0 | 0 | 0 |
| 5 | 5.6 | 7.5 |
| 10 | 12.0 | 16.7 |
| 15 | 17.0 | 24.0 |
| 20 | 22.1 | 31.4 |
(a) On the axes provided, construct an appropriately labeled graph of
this data.
(b) Based on the data and your graph from part (a), identify
compound X as either: a cofactor, a competitive inhibitor, or a
noncompetitive inhibitor of amylase. Justify your answer with
evidence from the data.
(c) Construct an additional line on your graph from part (a) that
represents your prediction as to the expected experimental results
at a temperature of 10° Celsius in the absence of compound X.
(d) Explain your prediction from part (c), stating why you placed the
additional line where you did.
▶️Answer/Explanation
Ans:
(a) 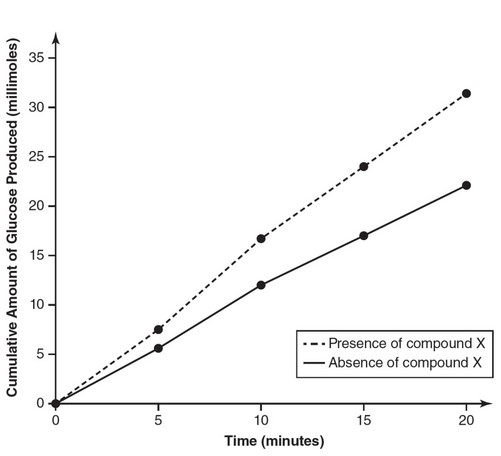
(b) Compound X is likely a cofactor. Cofactors increase enzyme
efficiency. Because the amount of product produced in the presence
of compound X is greater than the amount of product produced in
the absence of compound X, compound X is most likely a cofactor
of amylase.
(c) 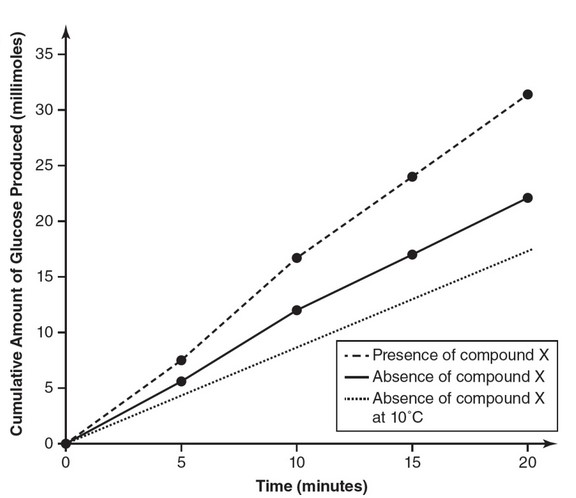
(d) At reduced temperatures, there are fewer molecular collisions
between the enzyme and the substrate as a result of the reduced
kinetic energy at lower temperatures. Therefore, it is reasonable to
predict that the reaction rate at 10°C would be slower than that of
either 37°C measurement and thus that line is lower on the graph
than the other two lines.
Question
Refer to glycogen metabolism in liver and skeletal muscle. A diagram of the intracellular processing of glycogen in liver and
muscle in response to adrenaline (epinephrine) follows.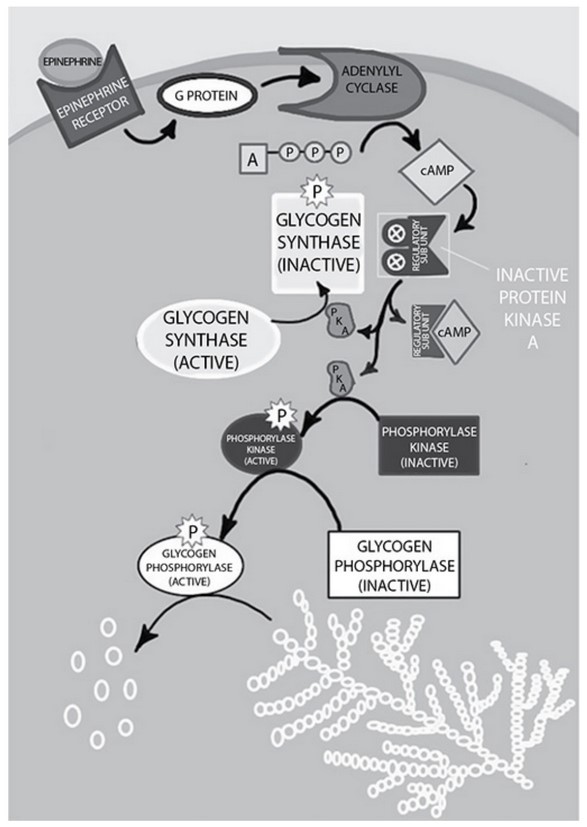
Liver and muscle both store glycogen in times of elevated blood glucose levels and can break it down later in order to increase blood
glucose levels (the liver) or during exercise (muscle). Both tissue types have enzymes for glycogen storage and enzymes of glycogen
synthesis present at the same time.
Explain how is possible for these enzymes of opposite function to coexist within the cell without performing a futile cycle of glycogen breakdown
and synthesis.
▶️Answer/Explanation
Ans:
Enzymes of opposite function can be controlled in a variety of ways.
Glycogen synthase and glycogen phosphorylase, the enzymes that synthesize
and break down glycogen, respectively, are regulated (in part) by
phosphorylation and dephosphorylation.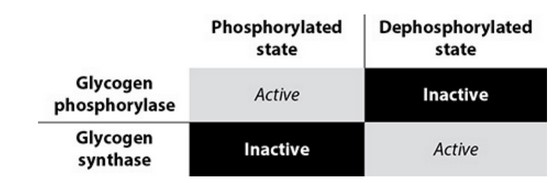
Epinephrine triggers the signaling cascade that results in the
phosphorylation of the two enzymes. Epinephrine is secreted in times of
stress and low blood glucose. The phosphorylation of the enzymes “turns on”
glycogen breakdown and “turns off” glycogen synthesis.
When the stress is over or blood glucose concentrations rise, the
phosphorylated state of the enzymes is not maintained, so the enzymes are in
the dephosphorylated state. This activates the synthesis of glycogen and
inhibits the breakdown.
