A. Elements
➢ All life forms made up of matter
- All matter made up of elements
■ Elements
● Substances that cannot be broken down into smaller substances by chemical means
- All matter made up of elements
B. Essential Elements of Life
➢ 96% of the mass of all living things made up of 4 elements:
- $\text{Oxygen (O)}$
- $\text{Carbon (C)}$
- $\text{Hydrogen (H)}$
- $\text{Nitrogen (N)}$
➢ Other elements (collectively 4% of biomass)
- $\text{Calcium (Ca)}$
- $\text{Phosphorus (P)}$
- $\text{Potassium (K)}$
- $\text{Sulfur (S)}$
- $\text{Sodium (Na)}$
- $\text{Chlorine (Cl)}$
- $\text{Magnesium (Mg)}$
➢ Trace elements
- $\text{Iron (Fe)}$
- $\text{Iodine (I)}$
- $\text{Copper (Cu)}$
C. Subatomic Particles
➢ Atom
- Smallest unit of an element
- Building blocks of physical world
➢ Subatomic Particles
- Protons
■ Packed with neutrons in nucleus
■ Positively charged
■ Most atoms have same amount of protons as electrons, making them electrically neutral - Neutrons
■ Packed with protons in nucleus
■ No charge
■ Isotopes
● Same element with different amount of neutrons in nucleus
● Vary in mass
● Radioactive isotopes decay spontaneously, giving off particles and energy - Electrons
■ Negatively charged
■ Spin around nucleus
■ Very small; effectively massless
■ Electrons on an atom differ in their amounts of potential energy
■ Electron’s state of potential energy is called its energy level, or electron shell
■ Valence electrons are those in the outermost shell, or valence shell
■ Chemical behavior of an atom is mostly determined by the distribution of electrons in electron shells
● Valence shell most important
● Elements with full valence shells are chemically inert
● Atoms with incomplete valence shells can share or transfer valence electrons with certain other atoms - Atoms of different various elements differ in number of subatomic particles
- $\text{Atomic number=protons in nucleus}$
- $\text{Mass Number= protons+neutrons}$
■ Average of all isotopes - Atomic mass+atom’s weighted average total mass

D. Compounds
➢ Compound occurs as result of 2 or more individual elements combining in a fixed ratio
- Different properties of individual elements
- Formed by chemical reaction
➢ Bonds that hold compounds together
- Ionic bonds
■ $\text{nonmetal+metal}$
■ One or more electrons is transferred from one atom to another
■ One atom loses electrons (becomes positively charged) while the other gains electrons (becomes negatively charged)
■ Results from attraction of two oppositely charged ions
■ Cation has a positive charge
■ Anion has a negative charge
■ Cation and anion form to create ionic bond - Covalent bonds
■ $\text{nonmetal+nonmetal}$
■ Molecule consists of 2 or more atoms held together by covalent bonds
■ Formed when electrons are shared between atoms
■ In nonpolar covalent bond, electrons are shared equally
■ In polar covalent bond, electrons are shared unequally
■ In a single covalent bond, one pair of electrons is shared
● Double covalent when 2 pairs are shared, etc.
■ Structural formula used to represent atoms and bonding
● Ex. $\text{H-H}$
■ Molecular formula abbreviates structural formula
● Ex. ${H_2}$
● Electronegativity is an atom’s attraction for the atoms in a covalent bond- The more electronegative an atom, the more strongly it pulls shared electrons toward itself
- Hydrogen bonds
■ Hydrogen atom covalently bonds to one electronegative ato is also attracted to another electronegative atom
■ In living cells, hydrogen bonds are usually oxygen or other nitrogen atoms
○ Van der Waals Interactions
■ Weakest
■ If electrons are distributed asymmetrically in molecules or atoms, they can result in “hot spots” of positive or negative charge
■ Attractions between molecules that are close together as a result of these charges
● How geckos climb
E. Water: The Versatile Molecule
➢ In water, electrons are not shared equally in the bonds between hydrogen and oxygen
- Hydrogen atoms have a partial positive charge while oxygen atoms has a partial negative charge
■ Water is polar

➢ Hydrogen bonds
- Weak attractions that result of water’s polarity
■ Positive end of another polar molecule attracted to oxygen negative charge, and vice versa with the hydrogen end
■ Hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom
■ Weak Individually, but strong on a larger scale
- Lends watermany special properties
■ Cohesion
● Tendency for water to stick to water
● Important during transpiration - Water evaporates, pulls other water molecules with it, pulling all the way down from leaves to roots
■ Adhesion
● Tendency of water to stick to other substances
● $\text{Cohesion + Adhesion =}$ capillary action - Allows water to flow up roots/trunks/branches of trees in thin vessels
■ Surface tension
● Results from cohesion of water molecules
● Ex. water striders can sit on top of water without sinking
■ High heat capacity
● Heat Capacity=ability of a substance to resist temperature changes
● Keeps ocean temperatures stable
● Allows organisms to keep constant body temperature, since most life
forms are mostly made up of water
● Heat is absorbed when hydrogen bonds break, released when hydrogen bonds form
■ High heat of vaporization
● Heat a liquid must absorb for 1g to be converted to gas
● Evaporative cooling - As a liquid evaporates, its remaining surface cools
■ How sweat works to cool body down
■ Expansion on freezing
● Lattice structure of ice causes water to expand on freezing
● Allows ice to float on top of lakes in winter - Animal life can live beneath ice
■ Versatility as a solvent
● Solution is a liquid that is a homogenous mix of substances
● Solvent is the dissolving agent of a solution
● Solute is the substance that is dissolved
● Aqueous solution is one where water is the solvent
● Polarity of water allows it to be a versatile solvent - Can form hydrogen bonds easily
● Hydrophobic substances do not dissolve in water, but hydrophilic ones will
F. Acids and Bases
➢ Solution is acidic if it contains a lot of $H^{+}$
➢ Solution is alkaline if it contains a lot of OH-
➢ Measured on pH scale
- Logarithmic
- Numbered 1-14
■ $\text{Acids 1-7 pH}$
■ $\text{Bases 7-14 pH}$
➢ Buffers maintain stable pH
G. Organic Molecules
➢ Organic compound contains Carbon
➢ Inorganic compound does not contain carbon
➢ Carbon often surrounded by hydrogen
➢ Carbon is a versatile atom
- Can bind with many elements
- Many “slots” to bind with elements
■ 4 valence electrons
● Can form 4 covalent bonds
■ Makes large, complex molecules possible - In molecules with multiple carbons, each carbon bonded to 4 other atoms has a tetrahedral shape
■ When 2 carbons are formed by a double bond, the atoms joined to the carbons are one the same plane as the carbons - Electron configuration gives it covalent compatibility with other elements
- Hydrocarbons consist of only carbon and hydrogen
■ Can undergo reactions that release a large amount of energy
■ isomers are compounds with the same molecular formula but different
structures/properties
● Usually only one isomer is biologically active - Functional groups are the components of organic molecules that are most commonly involved in chemical reactions
■ Number and arrangement of functional groups give each molecule its unique properties
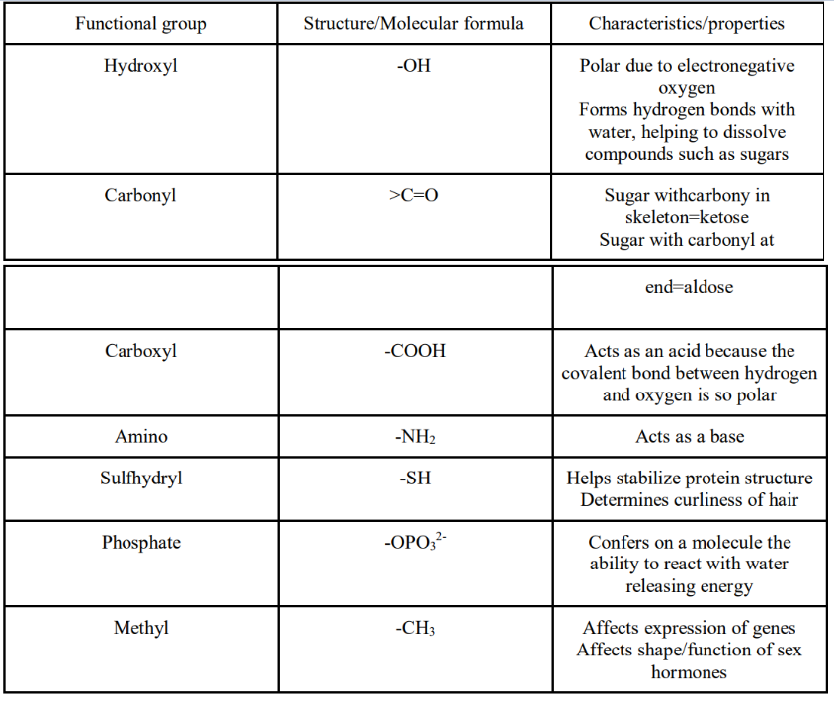
➢ Most macromolecules are chains of building blocks called polymers. The individual building blocks of a polymer are called monomers
➢ Carbohydrates
- Contain carbon, hydrogen, and oxygen in a 1:2:1 ratio
- Monosaccharides
■ Most common are glucose and fructose
● Glucose- Most abundant
- Part of food humans eat
- Made by plants during photosynthesis
■ Broken down to release energy
● Fructose- Common sugar in fruits
● Can be depicted as either straight or rings

■ 6 carbon-sugars
● Formula: $\mathrm{C}_6\mathrm{H}_{12}\mathrm{O}_6$
- Disaccharides
■ 1 monosaccharide+1 monosaccharide=1 Disaccharide
■ Formed by dehydration synthesis
● Aka condensation
● Hydrogen (-H) from one sugar combines with hydroxyl group (-OH) of another sugar molecule to create water as byproduct

● Bond is called glycosidic linkage
■ Broken apart by hydrolysis
● Reverse of dehydration
● Water is used to break apart glycosidic linkage
○ Polysaccharides
■ Repeated units of monosaccharides
■ Most common
● Starch
- Stores sugar in plants
- Made up of alpha-glucose molecules
● Cellulose - Made up of $\beta$-glucose molecules
- Chitin
■ Structural molecule in walls of fungi/arthropod exoskeletons
■ Used as surgical thread since it breaks down in body
● Glycogen
- Stores sugar in animals
➢ Proteins
- Amino acids=monomer of proteins
■ 20 kinds of naturally occurring amino acids - Contain:
■ Carbon
■ Hydrogen
■ Oxygen
■ Nitrogen - 4 parts of an amino acid centered around a central carbon
■ Amino group $(-NH_2)$
■ Carboxyl group (-COOH)
■ Hydrogen
■ R group
● Aka side chain
● Interchangeable
● Vary in composition, polarity, charge, shape depending on specific side chain
● Polar R groups point outward, hydrophobic R groups point inward - Polypeptides
■ Amino acid + amino acid= dipeptide
● Formed by dehydration synthesis
● Bond is called a peptide bond
● Multiple amino acids= polypeptide - Once a polypeptide chain twists and folds on itself, it forms a $\text3D$ structure called a protein

- Higher protein structure (4 levels total)
■ Primary structure
● Linear sequence of amino acids
● Covalent (peptide) bonds
■ Secondary structure
● Protein beings to twist–2 options
- Forms a coil (alpha-helix)
- Zigzagging pattern (known as beta-pleated sheets)
● Shape depends on R-group
● Formed by amino acids that interact with other amino acids closeby in the primary structure
● Hydrogen bonds between carbonyl and amino group
● Interactions between amino and carboxyl groups of protein backbone
● After secondary structure forms, formerly distant amino acids are now closeby–tertiary structure can form
■ Tertiary structure
● Can be both alpha and beta helix/sheets within structure
● Covalent disulfide bridge often stabilizes structure
● Bonds between R groups - Hydrogen bonds
- Ionic bonds
- Disulfide bridges
- Hydrophobic interactions
■ Quaternary structure
● Several different polypeptide chains sometimes interact with each other
● Same bonds as above, but between peptide chains rather than between R
groups
■ Mistakes in structure can denature a protein
● Change of shape=change of function - Ex. pH or heat can denature protein
■ Protein folding can involve chaperone proteins (chaperonins)
● Help protein fold properly
● Make process more efficient

➢ Lipids
- Like carbs, consist of carbon, hydrogen and oxygen, but not in a fixed ratio
- Do not form polymers
- Little-no affinity for water
■ Hydrophobic due to nonpolar covalent bonds of hydrocarbon - Common examples:
■ Triglycerides
● Glycerol molecule+3 fatty acid chains attached - Fatty acid chain is mostly a long chain of carbons where each carbon is covered in hydrogen; One end of the chain has a carboxyl group $(-COOH)$
■ Vary in length and /location(s) of double bonds - Glycerol is a 3-carbon alcohol with a hydroxyl group attached to each carbon
● Fats separate from water because water forms hydrogen bonds with itself while excluding the fats
● In order to be made, each of the carboxyl groups of the 3 fatty acids must react with one of the 3 hydroxyl groups of the glycerol molecule via dehydration synthesis - bond=ester linkage
● Saturated fatty acid - No double bond
- Carbon chain completely filled (“saturated”) with hydrogen
- Usually solid at room temp.
● Unsaturated fatty acid - Double bond along carbon chain, causing a bend
■ Bend allows triglyceride to become LESS dense, making it liquid at room temperature - Polyunsaturated fatty acid has multiple double bonds within the fatty acid, causing many bends

■ Phospholipids

➢ Nucleic Acids
- Contain carbon, hydrogen, oxygen, nitrogen, and phosphorous
- Structure
■ Nitrogenous base
■ Pentose sugar
■ Phosphate group
■ Portion of nucleotide w/o phosphate group is called nucleoside - Store, transmit, and help expres hereditary information
- monomer=nucleotides
- Amino acid sequence of a polypeptide is programmed by a unit of inheritance called a gene
■ Made up of DNA - Deoxyribonucleic acid (DNA)
● sugar=deoxyribose
● Contains genetic/hereditary information
● Provides directions for its own replication
● Directs synthesis of messenger RNA (mRNA), and through mRNA, controls protein synthesis - Occurs on ribosomes
- Ribonucleic acid (RNA)
● sugar=ribose
● Essential for protein synthesis - 2 families of nitrogenous bases
■ Pyrimidines
● Single 6-membered ring
● Ex. - Cytosine
- Thymine (only DNA
- Uracil (only RNA)
■ Purines
● 6-membered ring fused to a 5-membered ring
● Ex. - Adenine
- Guanine
- Nucleotide Polymers
■ Nucleotide polymers linked together to build a polynucleotide
■ Adjacent nucleotides are joined by covalent bonds that form between the$ -OH$ group on the 3’ carbon of one nucleotide and the phosphate on the 5’ carbon on the next
● Links create a backbone of sugar-phosphate units with nitrogenous bases as appendages
■ RNA molecules usually exist as single polypeptide chains
■ DNA molecules have 2 polynucleotides spiraling around an imaginary axis, forming a double helix
● Two backbones run in opposite 5’→3’ directions from each other (antiparallel)
● One DNA molecule contains many genes
● Nitrogenous bases pair up and form hydrogen bonds - Adenine-Thymine
- Guanine-Cytosine
- Complementary base pairing
- In RNA, thymine is replaced by uracil, so A and U pair



F. Origins of the Earth
➢ Alexander Oparin and J. B. S. Haldane proposed that the primitive atmosphere contained the
following gases:
- Methane $(CH_4)$
- Ammonia $(NH_3)$
- Hydrogen $(H_2)$
- Water$ (H_2O)$
- No free oxygen$ (O_2)$
■ No oxidation/reduction
■ Rocks do not release oxygen through weathering - Gases collided, producing chemical reactions that eventually led to the organic molecules
we know today - Substantial support until 1953
➢ 1953, Stanley Miller and Harold Urey simulated the conditions of primitive Earth in a lab,
- Put theoried gases into flask, struck them with electrical charges to simulate lightning, and organic compounds similar to amino acids appeared
➢ Current theory of the origin of life suggests 4 main stages - 1. Formation of amino acids
- 2. Monomers form polymers
- 3. Enclosure of small organic molecules into larger ones
- 4. Self-replicating molecules that can direct synthesis of other organic substances
■ Energy sources for early organic synthesis
● Lightning
● Volcanic eruptions
➢ RNA world hypothesis - Original life-forms were simple molecules of RNA
■ RNA not restricted to double helix
■ RNA capable of replicating and passing genes Complex organic compounds must have formed via dehydration synthesis
■ Organic compounds then used as food by cells
● Simple cells evolved into complex cells
➢ Heterotrophs
- living organisms that rely on organic molecules for food Aka consumers
➢ Autotrophs
- Organisms that make their own food
■ Most commonly via photosynthesis - Aka producers
