The Three States of Matter
- Gas: molecules/atoms have enough energy to move freely
- Particles so far apart from each other that intermolecular forces not considered
- Indefinite shape and volume
- Liquid: strong intermolecular forces and molecular motions
- Particles are always in contact but have enough energy to slide past each other
- Indefinite shape & definite volume
- Solid: strongest intermolecular forces, but the molecular motions are minimal
- Particles don’t have enough energy to move → always in contact and in fixed position
- Definite shape and definite volume
Characteristics
- Particles retain their chemical identity in all 3 states, but the volume, density, and interparticle distances are different
- Liquids & solids are incompressible (condensed state) & their density does not change with temperature
- These similarities are due to the molecules being close together in solids and liquids; far apart in gases
The Liquid State
- Liquids have low compressibility, lack of rigidity, and high density compared to gasses
- Surface tension: tendency of molecules to be pulled from the surface to the interior of a liquid; resistance of a liquid to an increase in its surface area
- Stronger InterMF = stronger surface tension b/c molecules resist being stretched/broken
- Viscosity: a measure of a liquid’s resistance to flow
- Capillary action: spontaneous rising of a liquid in a narrow tube
- Capillary action depends on the cohesive and adhesive forces present → during capillary action, the liquid molecules simultaneously adhere to the tubing while pulling each other up
- Cohesive forces: intermolecular forces among the molecules of the liquid
- Molecules attracted to same molecules
- Adhesive forces: forces between the liquid molecules and their container
- Molecules attracted to another type of molecule
- Cohesive forces: intermolecular forces among the molecules of the liquid
- Capillary action depends on the cohesive and adhesive forces present → during capillary action, the liquid molecules simultaneously adhere to the tubing while pulling each other up
Concave Meniscus Formed by Polar Water
- Water has both strong adhesive and cohesive forces, but bcuz the adhesive forces are stronger → water will have a concave meniscus (water is attracted to the glass)
- Nonpolar liquids have stronger cohesive than adhesive forces (not attracted to glass) → convex meniscus
Changes of State
- Phase Changes: when a substance changes from solid to liquid to gas
| Changes of State | Melting or Fusion |
Solid → Liquid | Melting (endo) |
Liquid → Solid | Freezing (exo) |
Liquid → Gas | Vaporization (endo) |
Gas → Liquid | Condensation (exo) |
Gas → Solid | Deposition (exo) |
Solid → Gas | Sublimation (endo) |
- Melting point: temp at which the substance goes from a solid to a liquid (or from a liquid to a solid)
- The strength of the InterMF determines the temp at which these phase changes will occur
- Boiling point: temp at which a substance goes from a liquid to a gas (or from a gas to a liquid)
- 2 Key Points
- At a substance’s MP or BP, two phases can exist simultaneously
- The temp of a substance does not change as the substance goes from one phase to another
- Only after all of the substance has changed phases does adding heat change the temp of the substance
- Heat of fusion:ΔHfus·: The enthalpy change that occurs at the melting point when a solid melts
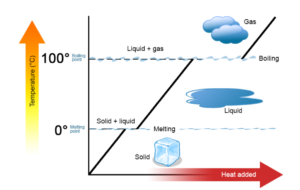
- When curve quickly changes slope to 0, all energy is used to overcome intermolecular forces holding the substance’s molecules together
Evaporation
- Evaporation: liquid becomes gas below a substances BP (occurs only for particles at the surface of a liquid)
- The particles with the highest KE can overcome the InterMF forces within the liquid and evaporate as gas
- Is a cooling process because the particles with the highest KE diffuse away from the liquid so the average KE of remaining particles decrease
Boiling
- Boiling: process by which a liquid becomes a vapor when it is heated to its boiling point
- As temp of liquid increases, vapor pressure increases until the vapor pressure of the liquid become equal to the surrounding atmospheric pressure → At this temp the liquid will boil (BP)
- As atmospheric pressure increase, BP of liquid increases
- The normal BP is the temperature at which the liquid boils at standard pressure
Evaporation vs Boiling
- Vaporization occurs in two ways: boiling and evaporation
- Evaporation is slower, occurs only from the surface of the liquid, does not produce bubbles, and leads to cooling.
- Boiling is faster, can occur throughout the liquid, produces lots of bubbles, and does not result in cooling.
Vapor Pressure and Changes of State
- Vapor Pressure: Liquid molecules at the surface escape into the gas phase → gas particles create pressure above the liquid in a closed container
- Gases are often collected over water so the vapor pressure of water must be subtracted from the total pressure in calculations
- Weaker IMF → Lower BP → will have higher vapor pressure before reaching boiling point
- Liquids are said to be volatile—they evaporate rapidly from an open dish
- Stronger IMF → higher BP/fewer molecules break away → will have lower vapor pressure before reaching boiling point.
- Heat of vaporization ΔHvap•:The energy required to vaporize 1 mole of a liquid at a pressure of 1 atm
- Water has high HoV so can absorb lots of heat and resist chemical change; needs lots of energy to freeze → cools air when it is warm and releases heat in the winter, stabilizes ocean temperature, climate
- Generally, the vapor pressure of a liquid is related to temperature and intermolecular forces
- Vapor pressure increases significantly with temperature.
- Temperature of the liquid increases = more molecules will have the minimum energy needed to overcome InterMF and escape into the vapor phase
- Vapor pressure increases significantly with temperature.
Phase Diagrams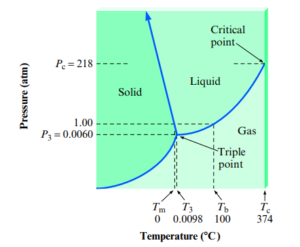
- Phase diagram: way of representing the phases of a substance as a function of temperature and pressure. (in a closed system)
- Lines represent phase changes
- Triple point: condition of temp and pressure where all three phases are present
- Critical temperature: temperature above which the vapor cannot be liquefied no matter what pressure is applied
- Critical pressure: pressure required to produce liquefaction at the critical temperature
- Critical point: critical temperature + critical pressure
Phase Diagram for Water
- Density and Phase Diagrams: the slope of the line between the solid and liquid region indicates which of these 2 phases is denser 1
- MP Curve has positive slope (/) → solid is denser
- Increasing pressure = increases melting point
- MP curve has negative slope (\)→ liquid is denser
- Increasing pressure = decreases melting point
- Water has a negative slope, but most other substances have a positive slope
- Increasing pressure = decreases melting point
