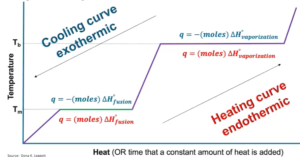
Energy of Phase Changes
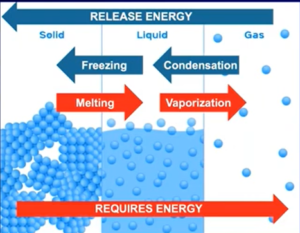
- → endothermic (atractive forces are being broken); ← exothermic (attractive forces are forming)
- Heat of fusion (
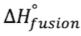 ) will be positive for melting and negative for freezing; heat of vaporization will be positive for vaporization and negative for freezing
) will be positive for melting and negative for freezing; heat of vaporization will be positive for vaporization and negative for freezing
- Heat of fusion (
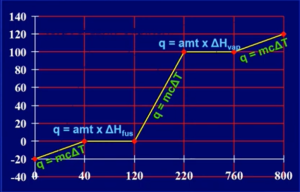
- Can’t use q = mcΔT during phase changes bcuz temp remains constant
- No change in KE, only change in PE
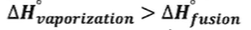
- During HoV, all the intermolecular forces/attraction are breaking but during HoF are only breaking/separating some
- Energy required for phase changes (ex: required to melt a substance)
- Heat to melting point: q = mcΔT
- Melting: q = (moles)(
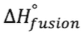 )
) - Add up values
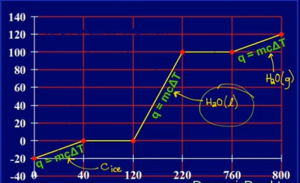
- Value of c will be different in each phase