Hess’s Law
- Hess’s Law: if you add chemical equations to get an overall equation, then you can also add the heat changes (ΔH) to get the overall heat change
- If two identical substances are on opposite sides of the arrow, they will cancel (reduce)
- If two identical substances are on the same side of the arrow, add the coefficients together
- Keep substances on the same side of the arrow in the final equation
- Ex:
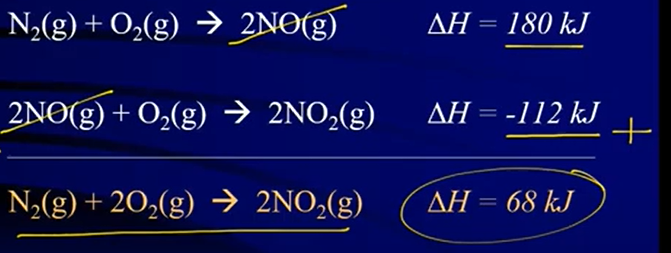
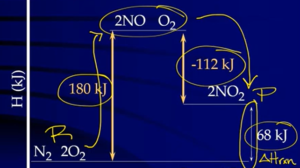
- Ex:
Calculations via Hess’s Law
1. Reverse any reactions as needed to get substances on correct side → If a reaction is reversed, (the sign of) ΔH is also reversed
2. If needed, multiply reactions by an integer to give the correct number of reactants and products → ΔH is multiplied by that same integer
- Tips: Start process with compounds that only appear once; once used one elementary step, cross through it bcuz not going to use it again
Laws of Thermodynamics
- Thermodynamics: study energy transformations
- Closed systems can reach equilibrium→ then system can no longer be used for work (matter and energy cannot be transferred between system and its surroundings)
- Open system: exchange energy and matter with environment
First Law: “Principle of Conservation of Energy”
- Energy can be transferred and transformed into a different form but it cannot be created or destroyed
- Forms include kinetic energy and potential energy
- So organisms must get all their resources from the environment
- The energy of the universe is constant
Internal energy
- Internal Energy (E) (energy of an object): the sum of kinetic and potential energies of a system
- Can be changed by a flow of work, heat or both
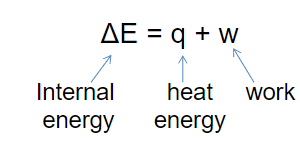
- ΔE = + internal energy of the system increased (temp increases)
- ΔE = – internal energy of the system decreased (temp decreases)
- Q = + heat flowed into the system
- Q = – heat flowed out of the system
- W = + the surroundings do work on the system (gas compression)
- W = – the system does work on the surrounding s (gas expands)
- To convert between L·atm and Joules, use 1 L·atm = 101.33 J
