Galvanic (Voltaic) Cells
- Galvanic cell = battery → provide power
- REDOX reactions can be used to change chemical energy into electrical energy
- Can use two half reactions and cause electrons to flow through a wire (electricity)
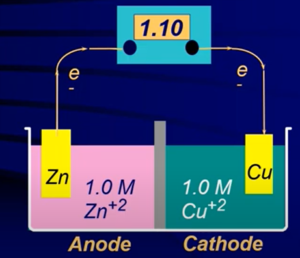
- With this set up (→), the system will start but then stop immediately BCUZ will have a build up of positive change in the left half cell and buildup of negative charge in right half cell
- For a circuit to flow, need a constant flow of charge
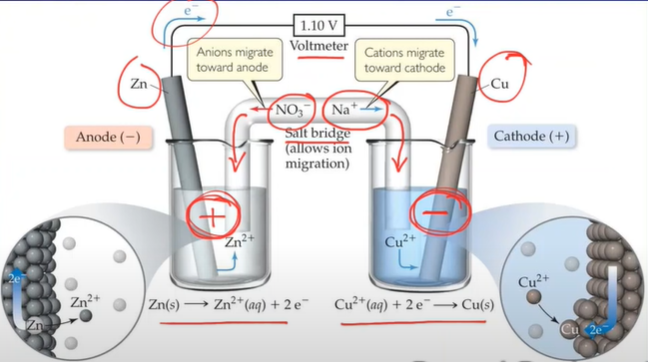
- Salt bridge fixes the above problem → allows (spectator; not part of redox reaction) ions to flow
- In the Anode solid atoms of zinc are being converted into aqueous zinc ions in beaker → zinc bar is losing mass over time
- Oxidation is taking place → the half cell is called the anode (electrons are being released)
- In the Cathode aqueous copper ions are being converted into solid copper which are deposited into the cathode → the mass of the electrode will increase over time
- Reduction is taking place → the half cell is called the cathode (electrons are being consumed)
- Electrons always flow from anode to cathode (“from A to C”)
- What if species involved in galvanic cell reaction are not solid (liquid, aqueous, or gas)?
- A solid electrode is still needed → usually platinum or graphite are used bcuz they are conductive but mostly unreactive
- Note: remove an electrode → voltage becomes zero and remains there
- A solid electrode is still needed → usually platinum or graphite are used bcuz they are conductive but mostly unreactive