Question
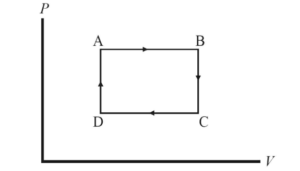
Which statement correctly characterizes the work done by the gas during the ABCA cycle shown in the above P-V diagram?
(A) There is no work done by the gas because the system both starts and concludes in state A.
(B) There is no work done because the work done during the transition from A→B cancels out the work done in transition from C→D.
(C) The work done by the gas is positive because the work done during the transition from A→B is greater than the work done in transition from C→D.
(D) The work done by the gas is positive because the work done during the transition from B→C is greater than the work done in transition from D→A.
▶️Answer/Explanation
Ans:C
Work is done when there is a change in volume. At constant pressure, the equation W = -PΔV tells us that when P is higher (as it is at path A→B) a greater amount of negative work is done on the gas than at lower P. Thus, the work done by the gas during the entire cycle is positive, and the constant volume paths have no influence on the amount of work.
Question
A thermodynamic process is conducted wherein an ideal gas is taken from State A to B to C to D to A. State A is at a pressure P and a volume 5V. State B is at pressure 4P and volume V. State C is at pressure 4P and volume 4V. State D is at pressure 2P and volume 10V. Which step in the process requires the largest change in internal energy of the system?
(A) State A to State B
(B) State B to State C
( C) State C to State D
(D) State D to State A
▶️Answer/Explanation
Ans: D
The internal energy is proportional to the temperature of the system, and the Ideal Gas Law explains that the product of pressure and volume is also proportional to the temperature. State A is at 5PV, State B is at 4PV, State C is at 16PV, and State D is at 20PV. The change from State D to State A requires the largest change in energy.
Questions
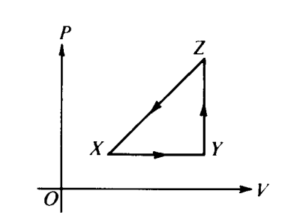
A thermodynamic system is taken from an initial state X along the path XYZX as shown in the PV-diagram.
Question(a)
For the process X→Y, ΔU is greater than zero and
(A) Q < 0 and W = 0 (B) Q < 0 and W > 0 (C) Q > 0 and W < 0 (D) Q > 0 and W > 0
▶️Answer/Explanation
Ans:C
Solution: For X ⇒ Y, the process is isobaric. Since the gas is expanding, W < 0 and since the temperature is increasing, ΔU > 0 and ΔU = Q + W so Q > 0 (it is also true because process XY lies above an adiabatic expansion from point X)
Question(b)
For the process Y→Z, Q is greater than zero and
(A) W < 0 & ΔU = 0 (B) W = 0 & ΔU < 0 (C) W = 0 & ΔU > 0 ( D) W > 0 & ΔU >0
▶️Answer/Explanation
Ans:C
Solution: For Y⇒Z, the process is isochoric, which means no work is done (W = 0) and since the temperature is increasing, ΔU > 0
