Question
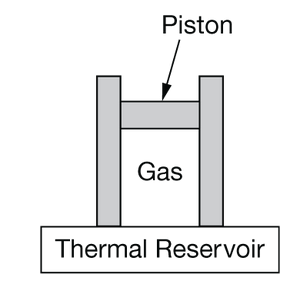
The figure shows a sample of an ideal gas enclosed within a cylinder that has been fitted with a movable piston. The piston and the sides of the cylinder are thermally insulated. The bottom of the cylinder is in contact with a thermal reservoir. The gas is compressed isothermally while thermal equilibrium is maintained with the reservoir. The piston, gas, and reservoir form a closed, isolated system. True statements about entropy for this reversible situation include which of the following? Select two answers.
A The entropy of the gas decreases because work is done on the gas while its temperature remains constant.
B The entropy of the reservoir increases because thermal energy is transferred to it from the gas.
C The system has a net increase in entropy as a result of the process.
D The change in entropy of the system depends on how quickly the isothermal compression occurs.
▶️Answer/Explanation
Ans:A , B
The temperature is constant, so the change in entropy is proportional to the change in thermal energy. Since thermal energy is being transferred from the gas to the reservoir, the thermal energy of the gas, and hence the entropy of the gas, is decreasing.
The temperature is constant, so the change in entropy is proportional to the change in thermal energy. Since thermal energy is being transferred from the gas to the reservoir, the thermal energy of the reservoir, and hence the entropy, is increasing.
Question
Which of the following describes the microscopic difference between the change in entropy of a gas during reversible processes (which are theoretical) and irreversible processes (which actually occur)?
▶️Answer/Explanation
Ans:C
Entropy is a measure of how many different microscopic ways a system may be arranged to give the same measurable macroscopic system quantities, such as volume, pressure, and temperature. Thus, entropy is often described as a measure of the disorder of a system.
Question
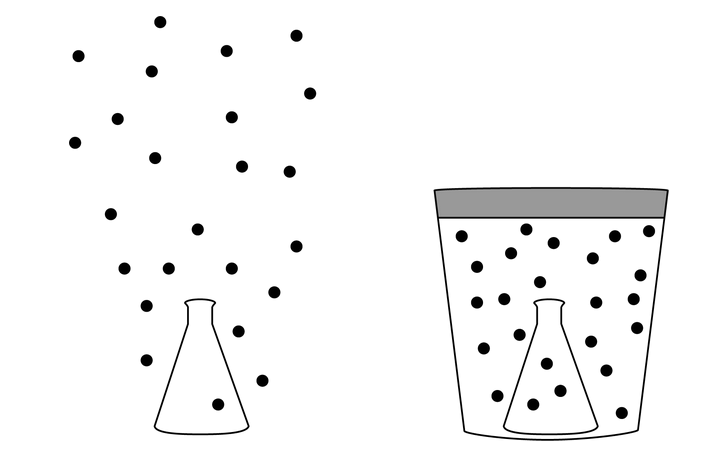
Two identical samples of helium gas are in identical sealed flasks at room temperature. One flask is just sitting in the room, and the other is inside a large, insulated vacuum container. Both flasks are opened and the samples are released, so the helium in one flask spreads throughout the room and the helium in the other flask spreads in the sealed container, as shown in the figures. Which of the following is true of the change in entropy that occurs in each case when the flasks are opened?
A The entropy increases in both cases because each system is now in a more disordered state.
B The entropy increases as the helium mixes with air but not as it spreads out into the sealed container.
C There is no change in entropy for either case because the temperature of the helium does not change.
D The entropy changes in both cases, but there is not sufficient information to determine whether it increases or decreases.
▶️Answer/Explanation
Ans:A
The helium that mixed with the air and the helium that spread out into the container are both more disordered than they were in their original states.
Question
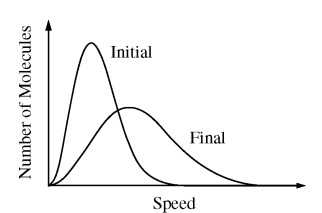
The graph above shows the initial and final molecular speed distributions of a gas as a result of a thermodynamic process. Which of the following processes could produce this change?
(A) Expansion of the gas at constant temperature
(B) Compression of the gas with no transfer of energy by heating
(C) Cooling of the gas at constant volume
(D) Cooling of the gas at constant pressure
▶️Answer/Explanation
Ans:B
Question
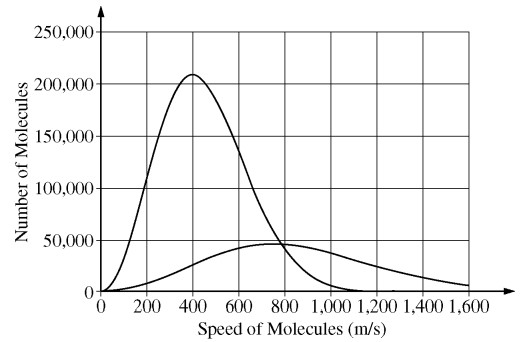
Two samples of the same type of gas molecules are placed in different closed containers and are thermally isolated from each other and the environment. The figure above shows the distribution of speeds for the molecules in each of the samples. The two samples are now mixed together and allowed to reach thermal equilibrium. Which of the following is true of the peak of the distribution for the mixed sample?
(A) It is closer to 400 m/s than it is to 800 m/s.
(B) It is closer to 800 m/s than it is to 400 m s .
(C) It is midway between the peaks of the distributions shown.
(D) There is not just one peak but two, at the same locations as the peaks of the distributions shown.
▶️Answer/Explanation
Ans:A
