Question
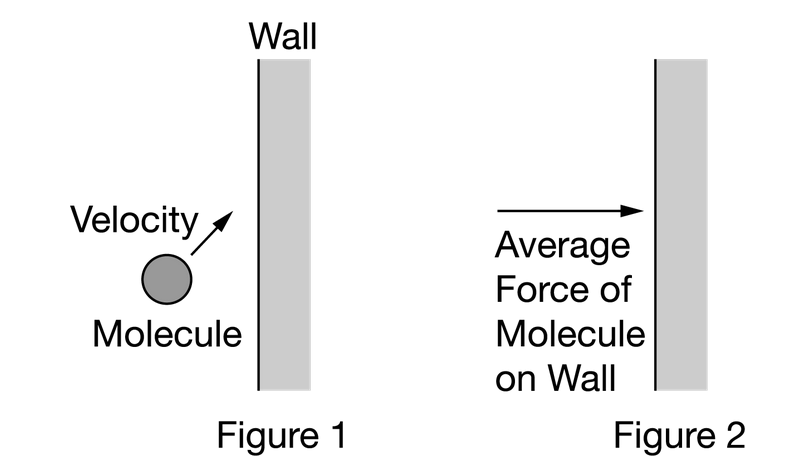
A gas is enclosed in a container. Figure 1 shows one gas molecule moving up and to the right toward a wall of the container. The molecule collides with the wall and afterward moves up and to the left. Figure 2 shows the average force exerted by the molecule on the wall. Which of the following best shows the average force exerted on the molecule during the collision? Gravity is negligible.
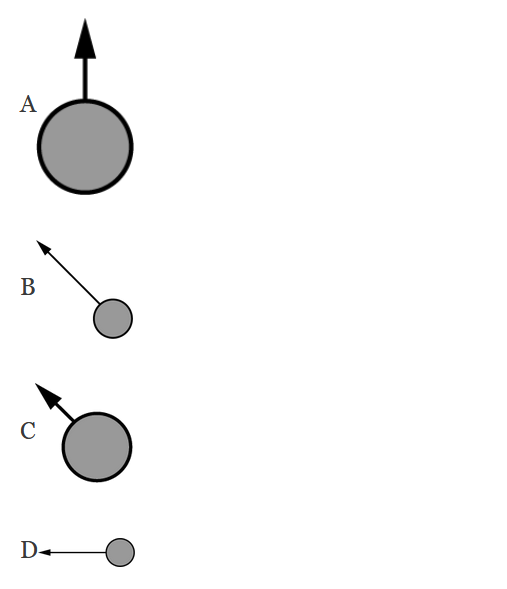
▶️Answer/Explanation
Ans:D
The force exerted by the molecule on the wall and the force exerted by the wall on the molecule form a Newton’s third law pair, so the force on the molecule is equal in magnitude and opposite in direction to the force that the molecule exerts on the wall.
Question
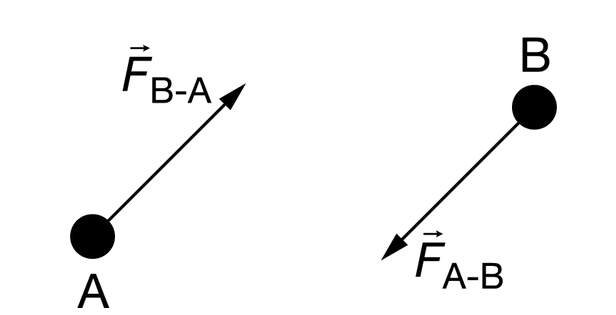
Two gas particles collide. Particle A has mass \(M_A\), and particle B has mass \(M_B\). When they collide, they exert forces on each other as shown in the diagrams, and particle A has an acceleration with magnitude \(a_A\) and particle B has an acceleration with magnitude \(a_B\). Which of the following correctly indicates the ratio \(\frac{a_A}{a_B}\) and whether the accelerations are in the same or the opposite directions?
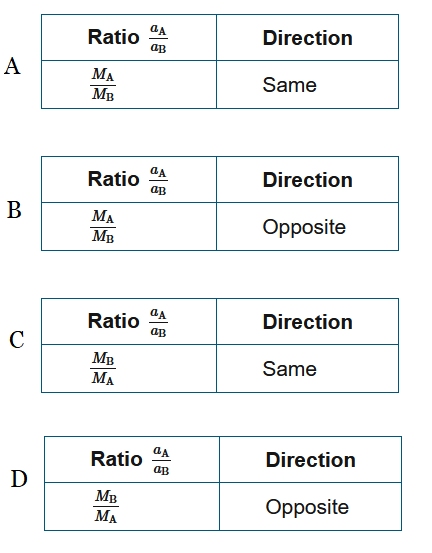
▶️Answer/Explanation
Ans:D
In a collision, a force is exerted on each object. The magnitude of that force is the same for both objects, but the direction of the force is different. According to Newton’s second law, the force is equal to the mass of the object times its acceleration. Therefore \(a_A=F_{B-A}/M_A\) , \(a_B=F_{A-B}/M_B\), and because \(F_{B-A}=F_{A-B}\), the ratio \(\frac{a_A}{a_B}=\frac{M_B}{M_A}\).
Question
An ideal gas is confined within a rigid container with a fixed lid. The container is placed on a heat source. Which of the following correctly compares the force exerted on the lid by the ideal gas and the force exerted on the ideal gas by the lid, and correctly relates the force to pressure?
A The force of the ideal gas on the lid is greater than the force of the lid on the ideal gas because of the increased kinetic energy of the ideal gas, and the net force causes an increase in the pressure.
B The force of the ideal gas on the lid is equal to the force of the lid on the ideal gas, but the force is increasing because of the increased kinetic energy of the ideal gas, leading to an increase in pressure.
C The force of the ideal gas on the lid is less than the force of the lid on the ideal gas, so the ideal gas does not lose as much kinetic energy, which reduces the pressure on the container.
D The force of the ideal gas on the lid is less than the force of the lid on the ideal gas because the velocity of the particles has increased, which lowers pressure according to the Bernoulli equation.
▶️Answer/Explanation
Ans:B
The force of the ideal gas on the container is the same as the force of the container on the ideal gas as described by Newton’s third law, since they are the two sides of the same interaction. There is a heat source, so the kinetic energy of the ideal gas will increase, thus increasing the change in speed of the ideal gas molecules when they collide with the walls of the container. The impulse-momentum theorem states that the greater change in speed comes from a larger force of the container on the ideal gas, and this larger force is equal and opposite to the force of the ideal gas on the container and leads to a greater pressure on the container.
Question
To launch a rocket, fuel in its engines is ignited, and the combustion produces gases at high pressure. As the combustion gases escape downward from one end of the rocket, the rocket is accelerated upward. Which of the following claims correctly describes forces in this situation?
A The combustion gas molecules collide with the atmospheric gas molecules. The atmospheric molecules exert an upward force on the combustion molecules, which allows the combustion molecules to exert a force on the rocket.
B The combustion gases are pushed out of the rocket because the pressure inside the engine is greater than the pressure outside the rocket. The rocket exerts a net downward force on the gases, which means the gases exert a net upward force on the rocket.
C The combustion gases are being pulled out by a force exerted by the atmospheric gas. This causes a force in the opposite direction on the combustion gases.
D As the combustion gases escape, the gas pressure inside the engines decreases. The atmospheric gas pressure is relatively constant, so the atmospheric pressure causes a force to be exerted on the rocket.
▶️Answer/Explanation
Ans:B
According to Newton’s third law, there will be a pair of forces acting between the rocket and the combustion gases. Because the gases are under pressure, the rocket exerts a force on the gases and the gases exert a force on the rocket.
Question
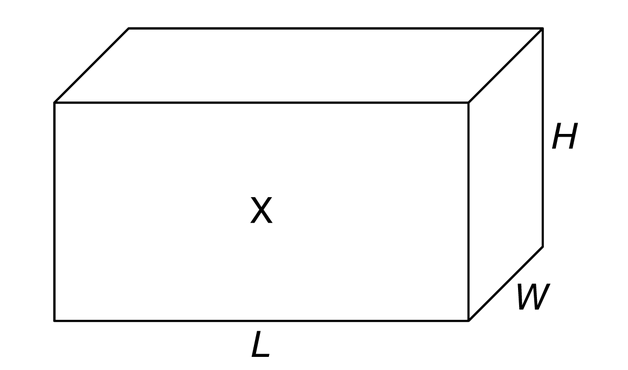
A room with length L, width W, and height H is filled with n moles of an ideal gas at temperature T, as represented by the figure. Which of the following indicates the magnitude and direction of the average force exerted on the gas by wall X ?
A \(nRT/H\) , into the page
B \(nRT/H\) , out of the page
C \(nRT/W\) , into the page
D \(nRT/W\) , out of the page
▶️Answer/Explanation
Ans:C
The wall exerts a force on the gas that is equal in magnitude and opposite in direction to the force exerted by the gas on the wall. The relevant quantities are related by the ideal gas law, \(P=\frac{nRT}{V}\) . The volume is \(LWH\), pressure is \(F/A\), and the area of wall X is \(LH\). So \(\frac{F}{LH}=\frac{nRT}{LWH}\), giving \(F=nRT/W\). The force exerted by the gas is directed out of the page, so the force exerted on the gas is into the page.
