Question: (12 points-suggested time 25 minutes)
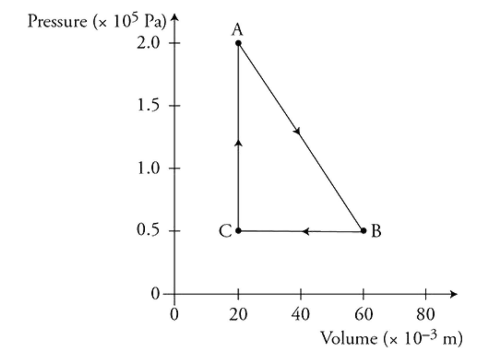
A mole of ideal gas is enclosed in a cylinder with a movable piston having a cross-sectional area of 1 x 10-2 m2. The gas is taken through a thermodynamic process, as shown in the figure.
(A) Calculate the temperature of the gas at state A, and describe the microscopic property of the gas that is related to the temperature.
(B) Calculate the force of the gas on the piston at state A, and explain how the atoms of the gas exert this force on the piston.
(C) Predict qualitatively the change in the internal energy of the gas as it is taken from state B to state C. Justify your prediction.
(D) Is heat transferred to or from the gas as it is taken from state B to state C? Justify your answer.
(E) Discuss any entropy changes in the gas as it is taken from state B to state C. Justify your answer.
(F) Calculate the change in the total kinetic energy of the gas atoms as the gas is taken from state C to state A.
(G) On the axis provided, sketch and label the distribution of the speeds of the atoms in the gas for states A and B. Make sure that the two sketches are proportionally accurate.
▶️Answer/Explanation
Ans:
Note that all the numbers in this problem are rounded to two significant digits because that is the accuracy of the data from the graph.
Part (A)
1 point-For the correct value of temperature with supporting equation and work: PV = nRT, T = 480 K.
1 point-For an explanation that the temperature of the gas is directly related to the average kinetic energy of the gas molecules.
Part (B)
1 point-For the correct force with supporting equation and work: F = PA = 2,000 N.
1 point-For an explanation of the mechanism that produces gas force on the piston.
For example: The gas molecules collide with the piston in a momentum collision that imparts a tiny force on the piston. The sum of all the individual molecular collision forces is the net force on the piston.
Part (C)
1 point-For indicating that the temperature of the gas is decreasing and explaining why this occurs.
For example: Since the PV value of the gas decreases, the temperature of the gas decreases in this process as indicated by the ideal gas law.
1 point-For indicating that the internal energy of the gas will decrease and explaining why this occurs.
For example: ΔU = nRΔT and the temperature is decreasing. Therefore, the internal energy of the gas also must decrease.
Part (D)
1 point-For indicating that the work in this process is positive because the process is moving to the left on the graph and that the thermal energy of the gas is decreasing because the temperature is decreasing.
1 point-For using the first law of thermodynamics to determine that heat is being removed from the gas.
For example: work is positive and the gas internal energy is decreasing. Therefore, using the first law of thermodynamics, ΔU = Q + W, we can see that heat must be leaving the gas during this process.
Part (E)
1 point-For indicating that the entropy of the gas is decreasing because thermal energy is being removed in this process. This reduces the spread of
the speed distribution of the gas, thus reducing disorder.
Part (F)
1 point-For the correct answer with supporting work:
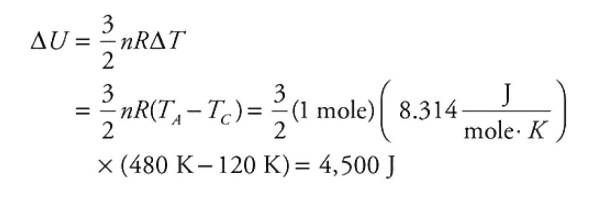
Part (G)
Based on the PV values, the temperature at point A is higher than that at point B. Thus, the peak for A must be at a higher speed than for B.
1 point-For both curves showing a roughly bell shape, and curve A having a higher average speed than curve B.
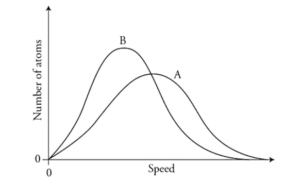
The area under the graphs must be equal because the number of molecules remains the same. This means the peak for A must be lower than that for B.
1 point-For curve A having a lower maximum than curve B, and both curves having roughly the same area beneath them.
Question: (12 points, suggested time 25 minutes)
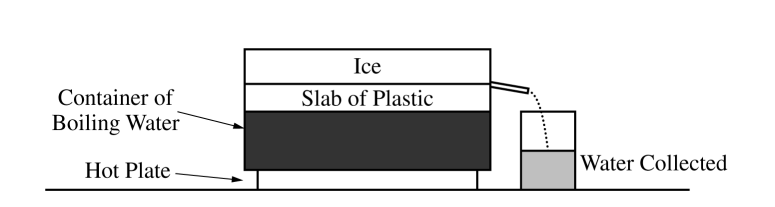
A group of students use the apparatus shown above to determine the thermal conductivity of a certain type of plastic. A hot plate is used to keep water in a container boiling at a temperature of 1000 C . They place a slab of the plastic with area 0.025 m2 and thickness 0.010 m above the container so that the bottom surface of the slab is at a temperature of 1000 C . They put a large block of ice with temperature 00 C on top of the plastic slab. Some of the ice melts, and the students measure the amount of water collected during a time Δt . The students correctly calculate the amount of energy Q delivered to the ice and thus determine Q /Δt . They repeat this experiment several times, each time adding an identical slab to increase the total thickness L of plastic. Their results are shown in the table below.
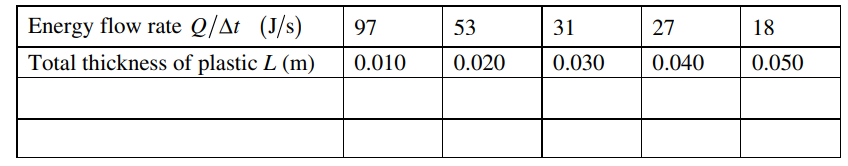
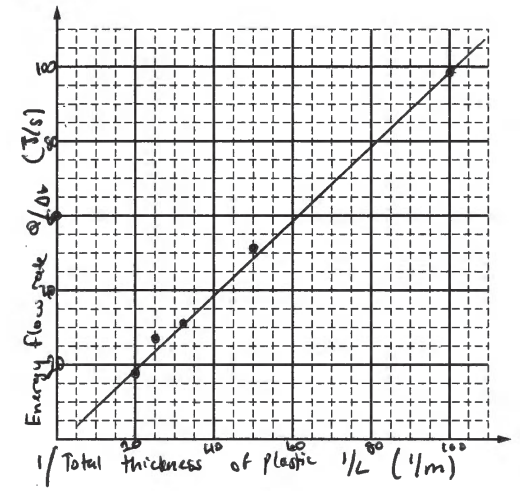
(a) The students want to create a graph to yield a straight line whose slope could be used to calculate the thermal conductivity of the plastic.
i. Label the axes below to indicate a pair of quantities that could be graphed to yield a straight line. Include units for the quantities.
ii. On the grid on the previous page, create a linear graph using the values for the quantities indicated in part (a)(i). Be sure to do the following.
• Add to the data table the values of any quantities to be plotted that are not already given.
• Scale the axes.
• Plot the data from the table.
• Draw a line that best represents the data.
iii. Use the graph to calculate the thermal conductivity of the plastic.
(b) Indicate one potential problem with the setup that could lead to an experimental value for the thermal conductivity that is different from the actual value. Use physics principles to explain the effect this problem could have on the experimental value.
(c) The rectangle below represents a side view of the plastic slab. Draw a single arrow on the diagram representing the direction of the net flow of energy through the plastic.

(d) Describe what occurs in the plastic at the microscopic level that explains the energy flow you indicated in part (c).
(e) An extra plastic slab sits on a wood surface, with both the plastic slab and the wood surface at room temperature. A student touches each and finds that the plastic slab feels cooler than the wood surface. Explain what causes this observation.
▶️Answer/Explanation
Ans:
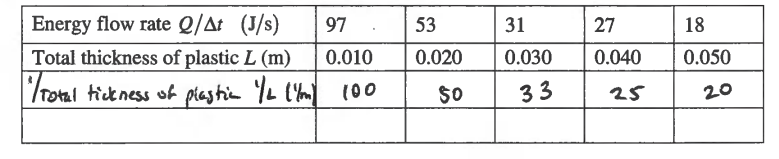
(a) i.
iii.
\(slope = \frac{QL}{\Delta t}=kA\Delta T\) \(k = \frac{1 J/S.m}{(0.025m^{2})(100^{\circ}C)}= \frac{1 J/S.m}{2.5m^{2}.^{\circ}C}\)
\(slope = \frac{79-59}{80-60}= 1 = \frac{QL}{\Delta t}\)
\(\therefore I = kA\Delta T\)
k = 1/A ΔT k = 0.4 J /ms 0C
(b)
The system is not isolated or sealed. When thermal energy is given off by the hot plate, some of the energy is lost to the surroundings instead of transferring to the slob of plastic. This decreases the change is temperature of the plastic and is turn the amount of ice that melted, giving a smaller thermal conductivity in the experiment.
(c)
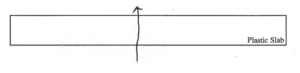
(d)
Thermal energy is given off by the hot plate, which this energy is transferred into the plastic as thermal energy. The particles become excited and gain energy. This energy is then transferred to the ice above it in thermal energy, thus heating the plastic.
(e)
Plastic is a good thermal conductor while wood is not. Thus plastic is more prone to temperature changes than wood is. This is why the plastic feels colder, because thermal energy is easier to flow in and out of the plastic In this case, the thermal energy left.
Question
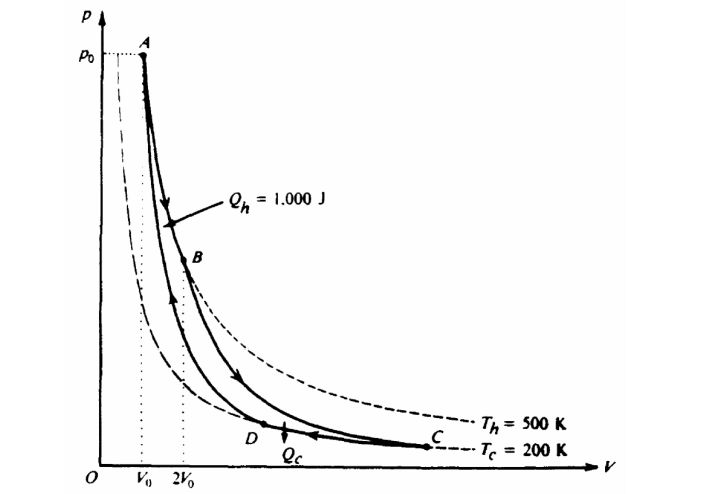
The pV-diagram above represents the states of an ideal gas during one cycle of operation of a reversible
heat engine. The cycle consists of the following four processes.
Process Nature of Process
AB Constant temperature ( Th = 500 K)
BC Adiabatic
CD Constant temperature ( Tc = 200 K)
DA Adiabatic
During process A B, the volume of the gas increases from Vo to 2Vo and the gas absorbs 1,000 joules of heat.
a. The pressure at A is po. Determine the pressure at B.
b. Using the first law of thermodynamics, determine the work performed on the gas during the process AB.
c. During the process AB, does the entropy of the gas increase, decrease, or remain unchanged? Justify your answer.
d. Calculate the heat Qc given off by the gas in the process CD.
e. During the full cycle ABCDA is the total work the performed on the gas by its surroundings positive, negative, or zero? Justify your answer.
▶️Answer/Explanation
Ans:
a. Since T is constant, pBVB = p0V0 and VB = 2V0 gives pB = ½ p0
b. ΔU = Q + W, since AB is isothermal, ΔU = 0 and W = –Q = –1000 J
c. The entropy of the gas increases because ΔS = Q/T and Q is positive (heat was added)
d. In a reversible (Carnot) engine \(\frac{Q_{H}}{Q_{C}} = \frac{T_{H}}{T_{C}} \) giving Qc = 400 J
e. Negative. In a clockwise cycle, the work done on the gas is negative. Or for the cycle Qnet = +600 J and ΔU =0 so W = –Q = – 600 J
