Question
Which of the following particles are not deflected by an electromagnetic field?
(A) Neutron
(B) Proton
(C) Positron
(D) Alpha particle
▶️Answer/Explanation
Ans:(A)
An object must have charge in order to interact with electromagnetic fields.
Question
Which of the following particles are not deflected by an electromagnetic field?
(A) Neutron
(B) Proton
(C) Positron
(D) Alpha particle
▶️Answer/Explanation
Ans:(A)
An object must have charge in order to interact with electromagnetic fields.
Question
Photons with energy of 8 eV are incident on a metal surface that emits electrons in a photoelectric effect experiment. If the emitted electrons can be stopped with a potential of 5 volts, the work function for this metal is at least
(A) 13 eV
(B) 8 eV
(C) 5 eV
(D) 3 eV
▶️Answer/Explanation
Ans:(D)
8 eV = W + KE
KE = 5 eV or less
W = 3 eV or more
Question
A doubly ionized helium atom is accelerated by a potential difference of 400 V. The maximum kinetic energy of the ion is equal to
(A) 400 eV
(B) 200 eV
(C) 1,600 eV
(D) 800 eV
▶️Answer/Explanation
Ans:(D)
Energy = qV = (2 e)(400 V) = 800 eV
Question
A scientist is using an electron microscope to study the structure of viruses ranging in size from 20 to 300 nm. The scientist must adjust the accelerating potential of the microscope to create an electron of the proper speed and wavelength to produce a detailed image of the virus. Which of the following graphs best depicts the relationship between accelerating voltage and the wavelength of the electrons?
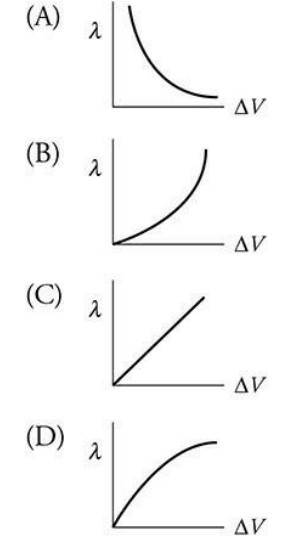
▶️Answer/Explanation
Ans:
A—The wavelength of a proton is given by: \(\lambda =\frac{h}{p}=\frac{h}{mv}\). Thus, the faster the electron is moving, the smaller its wavelength; the greater the accelerating potential, the higher the velocity will be. Therefore, as the accelerating potential increases, the wavelength decreases (an inverse relationship).
Question
In an experiment, monochromatic violet light shines on a photosensitive metal, which causes electrons to be ejected from the metal. Which of the following graphs best depicts the number of ejected electrons and the maximum energy of the ejected electrons versus the intensity of the violet light shining on the metal?
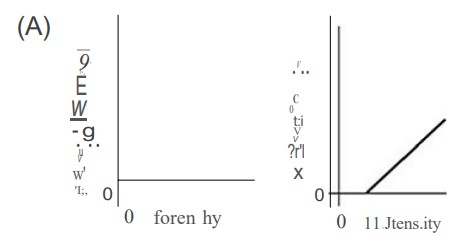
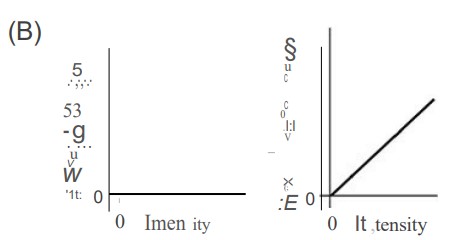
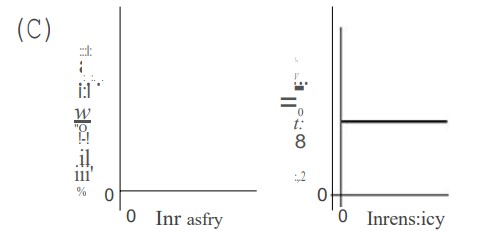
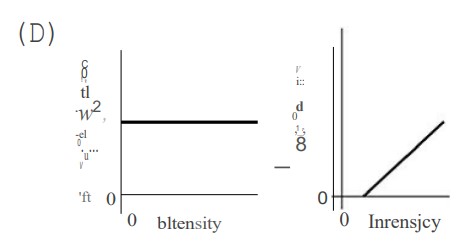
▶️Answer/Explanation
Ans:
C—Higher intensity (brighter light) simply means more photons. Therefore, higher-intensity light will eject more electrons. Lower intensity light ejects fewer electrons. When the intensity is zero, no electrons will be emitted. So we have answer choices B and C to choose from. The light is monochromatic (same wavelength and frequency); therefore, the maximum energy of each ejected photon is the same no matter how bright the light intensity is. Kqpq hf— J