Question
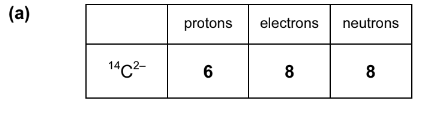
(a) Atoms and ions of elements are made up from the three subatomic particles, protons, electron and neutrons, in varying amounts. Complete the following table to show the number of each particle in \(^{14}C^{2-}\)

(b) Describe the observations you would make during the reactions, if any, of the following chlorides with water.
Write equations for any reactions that occur.
\(CCl _{4}\) observation
equation
\(GeCl_{ 4}\) observation
equation \(SnCl_{ 4}\) observation equation
(c) Suggest a reason for any difference in the reactivities of the chlorides given in (b).
(d) Use data from the Data Booklet to explain why an aqueous solution of \(SnCl _{2}\) reacts with\( Cl_{ 2}\)(g) but an aqueous solution of \(PbCl _{2}\) does not.
Write an equation for the reaction.
(e) (i) State the relationship between the Faraday constant and the Avogadro constant.
(ii) When a current of 1.2 A was passed through dilute sulfuric acid for 30 minutes, it was found that 130\( cm^{3}\) of oxygen, measured at 25 °C and 1 atm, was collected at the anode.The following reaction takes place.
\(2H_{2}O(l)\) → \(4H+(aq) + O_{2}(g) + 4e^{–}\)
Use these data and data from the Data Booklet to calculate a value for the Avogadro constant, L, by calculating
• the number of moles of oxygen produced,
• the number of moles of electrons needed for this,
• the number of coulombs passed,
• the number of electrons passed,
• the number of electrons in one mole of electrons (L).
Answer/Explanation
(b) \(CCl_{4}\): no reaction
\(GeCl_{4} \)and \(SnCl_{4}\): for each steamy fumes evolved or white solid produced
\(GeCl_{4} + 2H_{2}O \)→ \(GeO_{2} + 4HCl\)
\(SnCl_{4} + 2H_{2}O\) → \(SnO_{2} + 4HCl\)
(c) Ge/Sn use d–orbitals
or Ge /Sn have low lying d orbitals
or carbon cannot expand its octet
or carbon cannot accommodate more than 4 bonded pairs
(d) \( Sn^{4+} /Sn^{2+}\) = +0.15V and \(Pb^{4+} /Pb^{2+}\) = +1.69V and\( Cl_{2} /Cl^{–}\) = + 1.36V
\(Sn^{2+}\) is oxidised by \(Cl_{2}\) because its \(E^{\Theta}\) is less positive/more negative or\( Sn^{2+}\) is a good reducing agent due to its smaller E value than \(Cl_{2}\) ora
or\( Pb^{4+}\) is a stronger oxidising agent than \(Cl_{2}\) so \(Pb^{2+} \)with\( Cl^{2}\) reaction is not feasible or\( Sn^{4+}\) is a weaker oxidising agent than \(Cl_{2}\) so \(Sn_{2+}\) with\( Cl_{2}\) reaction is feasible
\(SnCl_{2} + Cl_{2}\) → \(SnCl_{4}\)
or\( Sn_{2}+ + Cl_{2}\) → \(Sn^{4+}\) + 2Cl^{–}\) or \(SnCl_{2} + Cl_{2} + 2H_{2}O\) → \(SnO_{2} + 4HCl\)
(e) (i) F = Le
(ii) moles of O2(g) = 130/24000 = \(5.417 x 10^{–3}\) mol
moles of electrons needed = 4 x 5.417 x 10–3 or 2.17 x \(10^{–2}\) mol
no. of coulombs passed = 1.2 x 30 x 60 or 2160 C
no. of electrons passed = 2160/ 1.6 x 10–19 or 1.35 x 1022
no. of electrons per mole = \(1.35 x 10^{22} / 2.17 x 10^{–2}\) =\( 6.2 x 10^{23} (mol^{–1})\)
Question
(a) The table shows information about some of the elements in the third period.
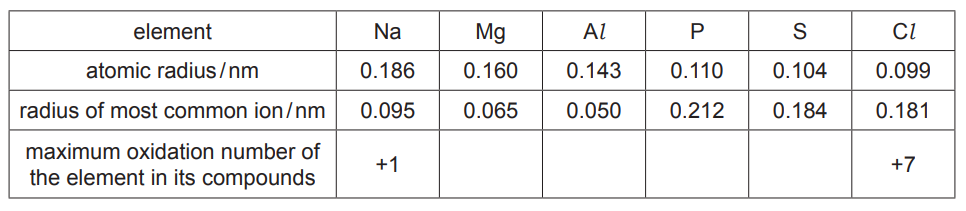
(i) Complete the table to show the maximum oxidation number of each element in its compounds.[1]
(ii) Explain why the atomic radius of elements in the third period decreases from $\mathrm{Na}$ to $\mathrm{Cl}$.[3]
(iii) The radius of the most common ion of $\mathrm{Mg}$ is much smaller than the radius of the most common ion of $\mathrm{S}$.
Identify both ions and explain the difference in their radii. [2]
(b) Phosphorus is a non-metal in the third period. It reacts vigorously with excess oxygen but slowly with chlorine.
Some reactions of phosphorus are shown
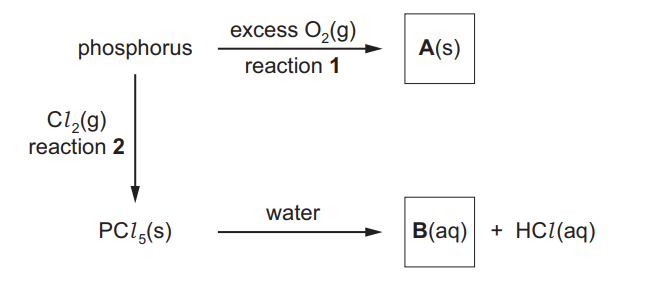
(i) Write an equation to represent reaction 1, the formation of compound A. ……………………………………………………………………………………………………………………… [1]
(ii) Give two observations you could make in reaction 2.
1. ………………………………………………………………………………………………………………………..
2. ………………………………………………………………………………………………………………………..[2]
(iii) Name compound B………………………………………………………………………………………………………………………. [1]
(c) Cerium is a lanthanoid metal that shows similar chemical reactions to some elements in the third period. Most of cerium’s compounds contain $\mathrm{Ce}^{3+}$ or $\mathrm{Ce}^{4+}$ ions.
(i) Cerium shows the same structure and bonding as a typical metal.Draw a labelled diagram to show the structure and bonding in cerium.[2]
(ii) Cerium(IV) oxide, $\mathrm{CeO}_2$, is a ceramic.
Suggest two physical properties of cerium(IV) oxide.
1.
5.[2]
(iii) A naturally occurring sample of cerium contains only four isotopes. Data for three of the isotopes are shown in the table.
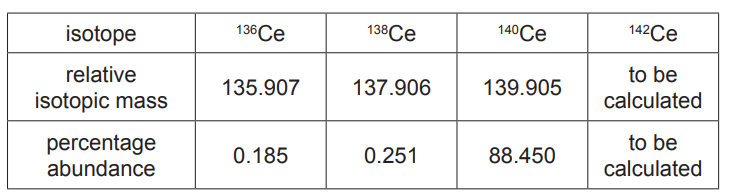
The $A_{\mathrm{r}}$ of the sample is 140.116 .
Use these data to calculate the relative isotopic mass of the fourth isotope in this sample of cerium.
Give your answer to three decimal places.relative isotopic mass $=$[3] [Total: 17]
▶️Answer/Explanation
Ans:
(a)(i)
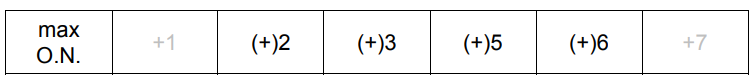
(a)(ii) (from Na to Cl) nuclear charge increases 1
electrons are in the same shell / have same shielding 1
greater/ stronger attraction (of electrons to nucleus)
(a)(iii) $\quad \mathrm{Mg}^{2+}$ AND $\mathrm{S}^{2-}$
(a)(iii) $\quad \mathrm{Mg}^{2+}$ AND $\mathrm{S}^{2-}$
ion of $\mathrm{Mg} / \mathrm{Mg}^{2+}$ has one fewer shell (than ion of $S / \mathrm{S}^{2-}$ )
(b)(i)
$\mathrm{P}_4+5 \mathrm{O}_2 \rightarrow \mathrm{P}_4 \mathrm{O}_{10} / 2 \mathrm{P}_2 \mathrm{O}_5$
(b)(ii) any 2 from:
• yellow/ green colour (of chlorine gas) disappears
• white flame
• white solid
• solid melts
(b)(iii) phosphoric(V) acid
(c)(i)
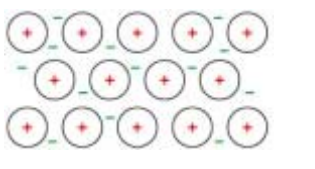
diagram showing regular arrangement of (positive) ions
surrounded by / sea of (delocalised) electrons
(c)(ii) any 2 from:
• high melting / boiling / sublimation point
• electrical /thermal insulator
• hard /rigid
• retains strength at high temperature / pressure
(c)(iii) M1
% abundance of fourth isotope
= 100 – (0.185 + 0.251 + 88.450) = 11.114
$\begin{aligned} & \text { M2 } \\ & \frac{(0.185 \times 135.907)+(0.251 \times 137.906)+(88.450 \times 139.905)+(11.114 \times \mathrm{RIM})}{100} \\ & =140.116 \\ & \therefore(140.116 \times 100)-12434.35=1577.246=11.114 \times \mathrm{RIM}\end{aligned}$
M3
$
\mathrm{RIM}=\frac{1577.246}{11.114}=141.915
$