Question
Titanium is a transition element in Period 4. It is commonly found as TiO2 in minerals.
(a) (i) Define transition element.
(ii) Identify two typical properties of transition elements.
1 …………………………………………………………………………………………………………………………
2 …………………………………………………………………………………………………………………………
(b) The \(TiO^{2+}\) ion forms when \(TiO_2\) reacts with an excess of sulfuric acid.
\(TiO^{2+}\) can be reduced by zinc metal in acidic conditions to form a purple solution containing \(Ti^{3+}(aq)\).
(i) \(TiO^{2+}(aq)\) is a colourless ion.
Suggest why.
(ii) Give the electronic configuration of an isolated \(Ti^{3+}\) ion.
\(1s^2\)
(iii) Write an ionic equation for the reduction of \(TiO^{2+}\) by zinc metal in acidic conditions.
(c) Acidified \(Ti^{3+}(aq)\) reacts with oxygen dissolved in water as shown.
\(4Ti^{3+} + O_2 + 2H_2O → 4TiO^{2+} + 4H^+\) \( ∆G^o = –436.1kJmol^{–1}\)
The standard reduction potential, \(E^o\), of \(O_2 + 4H^+ + 4e^– \leftrightarrow 2H_2O\) is +1.23V.
(i) Calculate the standard reduction potential, \(E^o\), in V, of the \(TiO^{2+}(aq)/Ti^{3+}(aq)\) half-cell.
Show your working.
\(E^o = ………………………… V\)
(ii) When aqueous citrate ions, \(C_6H_5O_7^{3–}\), are added to \(Ti^{3+}(aq)\), the \([Ti(C_6H_5O_7)_2]^{3–}(aq)\) complex forms.
Explain, in terms of d-orbitals, why \(Ti^{3+}\) is able to form complex ions.
(iii) Acidified \([Ti(C_6H_5O_7)_2]^{3–}(aq)\) does not react with oxygen dissolved in water, unlike acidified \(Ti^{3+}(aq)\).
Suggest what this means for the value of the standard reduction potential, \(E^o\) , of the following half‐cell.
\([Ti(C_6H_5O_7)_2]^{2–}(aq) + e^– \leftrightarrow [Ti(C_6H_5O_7)_2]^{3–}(aq)\)
Explain your answer.
(d) Some reactions of \(TiO_2\) are shown in Fig. 3.1.
The anion, \(acac^–\), is a bidentate ligand.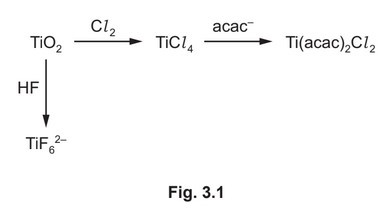
(i) The titanium ions in \(TiF_6^{2–}\) and \(Ti(acac)_2Cl_2\) have a coordination number of 6. State what is meant by coordination number.
(ii) Write an equation for the formation of \(TiF_6^{2–}\) from \(TiO_2\).
(iii) State what is meant by bidentate ligand.
(iv) \(Ti(acac)_2Cl_2\) shows both optical and geometrical (cis/trans) isomerism.
\(Ti(acac)_2Cl_2\) exists as three stereoisomers.
The structure of one stereoisomer of \(Ti(acac)_2Cl_2\) is shown in Fig. 3.2.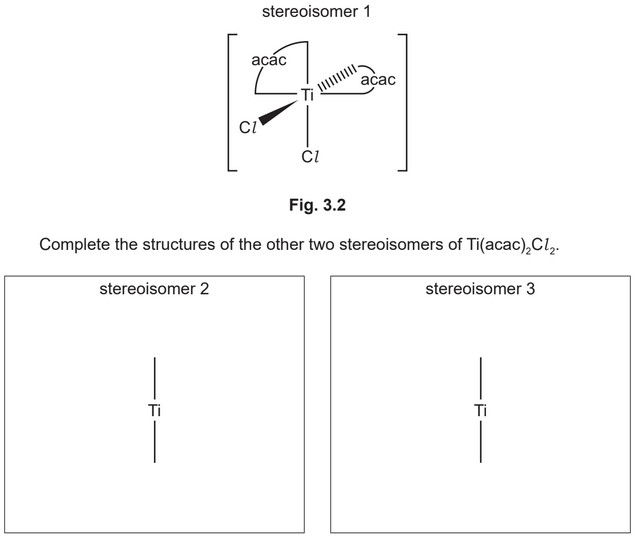
(v) The \(acac^–\) anion is symmetrical.
Deduce which, if any, of stereoisomers 1, 2 and 3 in (d)(iv) are polar.
Explain your answer.
Answer/Explanation
Answer:
(a) (i) (d-block) element that forms one or more stable ions with incomplete d subshell / incomplete d orbitals
(ii) • variable oxidation states
• behave as catalysts
• form complex ions / complexes
• form coloured compounds / ions
Any two for one mark
(b) (i) Ti is in +4 oxidation state so no d electrons / \(d^0\) OR Ti in \(TiO^{2+}\) has no d electrons / d0 [1]
cannot absorb photons / light in visible spectrum OR no wavelength / frequency absorbed in visible spectrum [1]
(ii) \((1s^2) 2s^2 2p^6 3s^2 3p^6 3d^1 (4s^0)\)
(iii) \(2TiO^{2+} + 4
H^+ + Zn
→ 2Ti^{3+} + 2H_2O + Zn^{2+}\)
(c) (i) M1: ∆G = \(– nE⦵_{cell}F\) AND n = 4
M2: ∴ \(E⦵_{cell}\) = –436100 / –4(96500) = 1.13 V ecf
M3: \(E⦵_{cell} = E^⦵(O_2,4H^+|H^2O) – E^⦵(TiO^{2+}|Ti^{3+}) = 1.23 – E^⦵(TiO^{2+}|Ti^{3+})\)
∴ \(E⦵(TiO^{2+}|Ti^{3+}) = (+)0.1 (V) ecf\)
(ii) \(Ti^{3+}\) empty / vacant d orbitals can form dative bonds / accept a lone pair from a ligand
OR \(Ti^{3+}\) has vacant d-orbitals which are energetically accessible
(iii) the E⦵ of the half-cell must be greater than +1.23 V / E⦵ of the \(O_2|H^+\) half-cell as E⦵cell< 0 and the reaction does not occur
(d) (i) the number of co-ordinate bonds being formed by the metal atom/ion
(ii) \(TiO_2 + 6HF → TiF_6^{2–} + 2H_2O + 2H^+\)
OR \(TiO_2 + 6HF → TiF_6^{2–} + 2H_3O^+\)
(iii) species with two lone pairs (of electrons) [1]
that form dative covalent / co-ordinate bonds to a central metal atom/ion [1]
(iv) 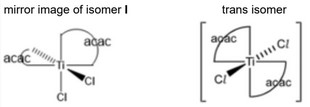
(v) isomer I AND cis isomer drawn by candidate ecf [1]
dipoles do not cancel / partial charges do not cancel [1]
Question
Iodine is found naturally in compounds in many different oxidation states.
(a) Iodide ions, \(I^–\), react with acidified \(H_2O_2(aq)\) to form iodine, \(I_2\), and water.
This reaction mixture is shaken with cyclohexane, \(C_6H_{12}\), to extract the \(I_2\). Cyclohexane is immiscible with water.
(i) Identify the role of \(H_2O_2(aq)\) in its reaction with \(I^–\) ions in acidic conditions.
Write an ionic equation for the reaction.
role
ionic equation
(ii) \(15.0cm^3\) of \(C_6H_{12}\) is shaken with \(20.0cm^3\) of an aqueous solution containing \(I_2\) until no further change is seen.
It is found that 0.390g of \(I_2\) is extracted into the \(C_6H_{12}\).
The partition coefficient of I2 between \(C_6H_{12}\) and water, Kpc, is 93.8.
Calculate the mass of \(I_2\) that remains in the aqueous layer.
Show your working.
mass of \(I_2\) in aqueous layer = ………………………… g
(iii) Suggest how the value of \(K_{pc}\) of \(I_2\) between hexan-2-one, \(CH_3(CH_2)_3COCH_3\), and water
compares to the value given in (a)(ii).
Explain your answer.
(b) The Group 1 iodides all form stable ionic lattices and are soluble in water.
(i) Define enthalpy change of solution.
(ii) Use the data in Table 1.1 to calculate the enthalpy change of solution of potassium iodide, KI.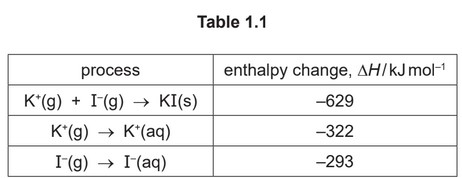
enthalpy change of solution = ………………………… \(kJmol^{–1}\)
(iii) Suggest the trend in the magnitude of the lattice energies of the Group 1 iodides, LiI, NaI, KI.
Explain your answer.
(c) The concentration of \(Cu^{2+}\)(aq) in a solution can be determined by the reaction of \(Cu^{2+}\) ions with I– ions.
reaction 1 \(2Cu^{2+}+ 4I^– → 2CuI + I_2\)
The \(I_2\) produced in reaction 1 is titrated against a solution containing thiosulfate ions, \(S_2O_32^–\), using a suitable indicator.
reaction 2 \(2S_2O_3^{2–} + I_2 → S_4O_6^{2–} + 2I^–\)
(i) \(A 25.0cm^3\) portion of a \(Cu^{2+}(aq)\) solution reacts with an excess of \(I^–\) (aq).
The end-point of the titration occurs when \(22.30cm^3\) of \(0.150moldm^{–3}\) \(S_2O_3^{2–}(aq)\) is added.
Calculate the concentration of \(Cu^{2+}\)(aq) in the original solution.
concentration of \(Cu^{2+}(aq)\) = ………………………… \(moldm^{–3}\)
(ii) Identify a suitable indicator for the titration.
(iii) Copper(I) and copper(II) both contain electrons in all five 3d orbitals.
Sketch the shape of a \(3d_{xy}\) orbital on the axes provided.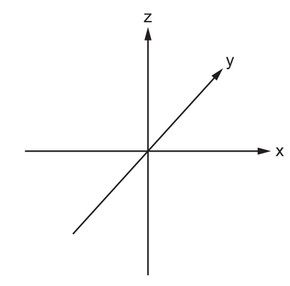
(d) The reaction of \(I^–\) ions with persulfate ions, \(S_2O_8^{2–}\), can be catalysed by \(Fe^{3+}\) ions.
\(2I^– + S_2O_8^{2–} → I_2 + 2SO_4^{2-}\)
Write equations to show how \(Fe^{3+}\) catalyses this reaction.
(e) An orange precipitate of \(HgI_2\) forms when \(Hg^{2+}\) ions are added to KI(aq).
The solubility of \(HgI_2\) at 25°C is \(1.00 × 10^{–7} gdm^{–3}\).
Calculate the solubility product, \(K_{sp}\), of \(HgI_2\).
Include units in your answer.
\([M_r: HgI_2, 454.4]\)
value of \(K_{sp}\) = …………………………
units = …………………………
Answer/Explanation
Answer:
(a) (i) oxidising agent [1]
\(H_2O_2 + 2H^+ + 2I^– → 2H_2O + I_2\) [1]
(ii) M1: \(K_{pc}\) (93.8) = [\(I_2\)(cyclohexane)] ÷ [\(I_2\)(aq)]
93.8 = (0.390 / 15) ÷ (x / 20)
M2: mass of \(I_2(aq)\), x \(= 5.54 × 10^{–3}\) (g) ecf
(iii) • \(K_{pc}\) would be lower
• hexan-2-one is more polar (than cyclohexane)
OR hexan-2-one is polar AND cyclohexane is non-polar
• \(I_2\) is (therefore) less soluble in hexan-2-one
All three correct for two marks
(b) (i) enthalpy change when one mole of a solute AND dissolves in water to form a solution of infinite dilution
(ii) –(–629) + ( –322) + ( –293) = (+)14 (kJ \(mol^{–1}\))
(iii) (cationic) charge density decreases \(Li^+\) to \(K^+\) [1]
so lattice energies become less negative / less exothermic AND because less attraction between ions [1]
(c) (i) M1: moles of thiosulfate \(= 0.02230 × 0.150 = 3.345 × 10^{–3}\)
\(M2: [Cu^{2+}] = 2 × 1⁄2 × 3.345 × 10^{–3} ÷ 0.0250 = 0.134 (mol dm^{–3}) ecf\)
(ii) starch
(iii) 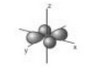
\(3d_{xy}\)
(d) Reduction of \(Fe^{3+}: 2Fe^{3+} + 2I^– → 2Fe^{2+} + I_2\) [1]
Regeneration of \(Fe^{3+}: 2Fe^{2+} + S_2O_8^{2–} → 2Fe^{3+} + 2SO_4^{2–}\) [1]
(e) \(M1: ([Hg^{2+}]) = 1.00 × 10^{–7} ÷ 454.4 = 2.20 × 10^{–10} (mol dm^{–3})\)
\(M2: K_{sp} = [Hg^{2+}][I^–]^2 = 4[Hg^{2+}]^3 = 4.26 × 10^{–29} ecf\)
\(M3: units = mol^3 dm^{–9} ecf