Question
(a) Successive ionisation energies for the elements magnesium to barium are given in the table
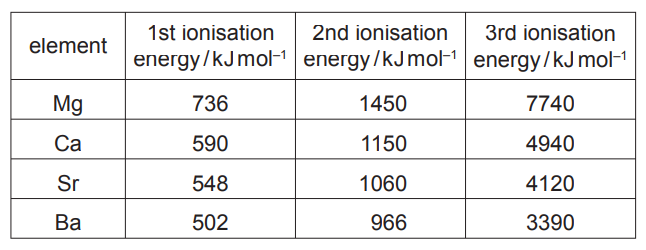
(i) Explain why the first ionisation energies decrease down the group.
……………………………………………………………………………………………………………………… [3]
(ii) Explain why, for each element, there is a large increase between the 2nd and 3rd ionisation energies.
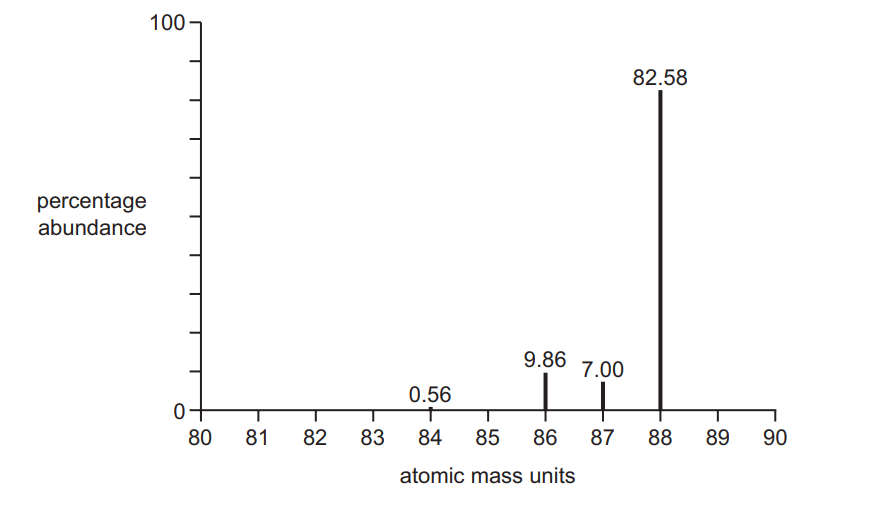
(b) A sample of strontium, atomic number 38, gave the mass spectrum shown. The percentage abundances are given above each peak.
(i) Complete the full electronic configuration of strontium.
$1 s^2 2 s^2 2 p^6$
(ii) Explain why there are four different peaks in the mass spectrum of strontium. [1]
(iii) Calculate the atomic mass, $A_r$, of this sample of strontium.
Give your answer to three significant figures.
$A_{\mathrm{r}}=$
(c) A compound of barium, $\mathbf{A}$, is used in fireworks as an oxidising agent and to produce a green colour.
(i) Explain, in terms of electron transfer, what is meant by the term oxidising agent. [1]
(ii) A has the following percentage composition by mass: $\mathrm{Ba}, 45.1 ; \mathrm{Cl}, 23.4 ; \mathrm{O}, 31.5$.
Calculate the empirical formula of $\mathbf{A}$.
empirical formula of $\mathbf{A}$ [3]
(d) Some reactions involving magnesium and its compounds are shown in the reaction scheme below.
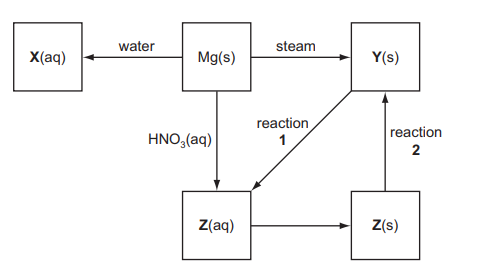
(i) Give the formulae of the compounds $\mathbf{X}, \mathbf{Y}$ and $\mathbf{Z}$.
$\mathbf{x}$
$\mathbf{Y}$
Z [3]
(ii) Name the reagent needed to convert $Y(\mathrm{~s})$ into $Z(\mathrm{Zq})$ in reaction 1 and write an equation for the reaction.
reagent
equation [2]
(iii) How would you convert a sample of $\mathrm{Z}(\mathrm{s})$ into $\mathrm{Y}(\mathrm{s})$ in reaction 2 ?
[1]
$\mathrm{Mg}$ to $\mathbf{X}$
$Z$ to $Y$
▶️Answer/Explanation
Ans:
(a) (i) increasing distance of (outer) electron(s) from nucleus
OR increasing distance of outer/ valence shell from nucleus
increased shielding/ screening (from inner shells)
reduces attraction
(ii) ( $3^{\text {rd }}$ electron for each in) inner/lower energy level/shell/closer to nucleus (than first two)/less shielding
(large) increase in nuclear attraction
(b) (i) $\left(1 s^2 2 s^2 2 p^6\right) 3 s^2 3 p^6 3 d^{10} 4 s^2 4 p^6 5 s^2$
(ii) four isotopes owtte
(iii) $\begin{aligned} & \frac{(84 \times 0.56)+(86 \times 9.86)+(87 \times 7)+(88 \times 82.58)}{100} \\ & =87.7 \text { (must be } 3 \text { sig figs) }\end{aligned}$
(c) (i) (a species that) gains / takes electron(s)
(ii) $\begin{array}{ccc}\mathrm{Ba} & \mathrm{Cl} & \mathrm{O} \\ \frac{45.1}{137} & \frac{23.4}{35.5} & \frac{31.5}{16} \\ \frac{0.329}{0.329} & \frac{0.659}{0.329} & \frac{1.969}{0.329} \\ 1.00 & 2.00 & 5.98 / 6 \\ \text { emp form }=\mathrm{BaCl}_2 \mathrm{O}_6\end{array}$
(d) (i)
$
\begin{aligned}
& \mathbf{X}=\mathrm{Mg}(\mathrm{OH})_2 \\
& \mathbf{Y}=\mathrm{MgO} \\
& \mathbf{Z}=\mathrm{Mg}\left(\mathrm{NO}_3\right)_2
\end{aligned}
$
\begin{tabular}{rl}
(ii) & reagent = nitric acid \\
& $\mathrm{MgO}+2 \mathrm{HNO}_3 \rightarrow \mathrm{Mg}\left(\mathrm{NO}_3\right)_2+\mathrm{H}_2 \mathrm{O}$ \\
(iii) Heat/thermal decomposition
$
\text { (iv) } \begin{aligned}
& \mathrm{Mg}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Mg}(\mathrm{OH})_2+\mathrm{H}_2 \\
& 2 \mathrm{Mg}\left(\mathrm{NO}_3\right)_2 \rightarrow 2 \mathrm{MgO}+4 \mathrm{NO}_2+\mathrm{O}_2
\end{aligned}
$
Question
The elements in Group 2, and their compounds, show many similarities and trends in their properties.
(a) Magnesium, calcium, strontium and barium all react with cold water.
(i) Describe what you would see when some calcium is added to cold water.[3]
(ii) Write an equation for the reaction taking place in (i).[1]
(iii) Describe how the reaction of barium with cold water would differ from the reaction of calcium in (i) in terms of what you would see.[1]
(b) Magnesium oxide can be formed by the reaction of magnesium and oxygen in the air.
(i) Draw a fully labelled reaction pathway diagram for the reaction between magnesium and oxygen.
(ii) Explain why there is no visible reaction when a piece of magnesium ribbon is exposed to the air
(iii) Magnesium oxide is used to manufacture heat-resistant bricks for furnace linings in the steel-making industry.
State and explain the property of magnesium oxide that makes it suitable for this use.[2]
(iv) Suggest a reason why magnesium oxide cannot be used as a lining for any furnaces containing acidic materials.[1]
(c) The nitrates and carbonates of the Group 2 elements, from magnesium to barium, decompose when heated.
(i) State the trend in the temperature of thermal decomposition of these Group 2 nitrates and carbonates.[1]
(ii) Give the equation for the thermal decomposition of magnesium carbonate.[1]
(iii) Give the equation for the thermal decomposition of calcium nitrate.[1][Total: 15]
▶️Answer/Explanation
Ans:
(a) (i) bubbles / effervescence/ fizzing
calcium gets smaller/ disappears
water turns cloudy / milky
calcium sinks
(ii) $\quad \mathrm{Ca}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+\mathrm{H}_2$
(iii) faster bubbling/ disappearance of Ba
OR
no/ less precipitate forms (owtte)
(b) (i)
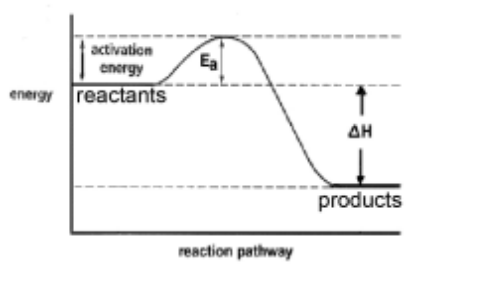
M1 – general layout with products below reactants AND both labelled M2 $-E_a$ and $\Delta H /$ energy change/released labelled with vertical lines
(ii) activation energy is high so few/no particles with $E \geqslant E_{\mathrm{a}}$
(iii) high melting/ boiling point
strong forces (of attraction/between oppositely charged ions)/ strong (ionic) bonding
(iv) MgO is basic / reacts with acid
(c) (i) increases (down the group)
(ii) $\mathrm{MgCO}_3 \rightarrow \mathrm{MgO}+\mathrm{CO}_2$
(iii) $2 \mathrm{Ca}\left(\mathrm{NO}_3\right)_2 \rightarrow 2 \mathrm{CaO}+4 \mathrm{NO}_2+\mathrm{O}_2$