Question
Ammonia can undergo an acid-base reaction with hydrogen chloride to form ammonium chloride. Which statement is correct?
A The ammonium ion is basic.
B The hydrogen atom from $\mathrm{HCl}$ donates a lone pair of electrons to the nitrogen atom.
C The $\mathrm{H}-\mathrm{N}-\mathrm{H}$ bond angle in ammonia is the same as the $\mathrm{H}-\mathrm{N}-\mathrm{H}$ bond angle in the ammonium ion.
D The $\mathrm{H}-\mathrm{N}-\mathrm{H}$ bond angle in the ammonium ion is the same as the $\mathrm{H}-\mathrm{C}-\mathrm{H}$ bond angle in methane
▶️Answer/Explanation
Ans:D
Question
With which compound does concentrated sulfuric acid react both as a strong acid and as an oxidising agent?
A magnesium carbonate
B potassium chloride
C sodium bromide
D sulfur trioxide
▶️Answer/Explanation
Ans:C
Question
Astatine, At, is below iodine in Group 17 of the Periodic Table.
Which statement is most likely to be correct?
A AgAt(s) reacts with an excess of dilute aqueous ammonia to form a solution of a soluble complex.
B Astatine and KCl(aq) react to form KAt(aq) and chlorine.
C KAt(aq) and dilute sulfuric acid react to form HAt(g).
D NaAt(s) and concentrated sulfuric acid react to form astatine.
▶️Answer/Explanation
Ans:D
Question:
The element astatine, At, is below iodine in Group 17 of the Periodic Table. Which statements concerning At are likely to be correct?
1 It is a dark-coloured solid at room temperature.
2 It is a more powerful oxidising agent than iodine.
3 Its hydride is more thermally stable than HBr.

▶️Answer/Explanation
Ans:D
Question
Some uses of chlorine and bromine are given.
Which is a use of bromine?
A making bleaches for textiles and the paper industry
B making CFCs
C making flame retardants and fire extinguishers
D making the polymer PVC
▶️Answer/Explanation
Ans:C
Question
Hydrogen chloride gas and hydrogen iodide gas have different thermal stabilities. The difference is due to a difference in the energies of some of the covalent bonds that are involved in the
decomposition.
Which row identifies the more stable of the two compounds, and gives the correct explanation?
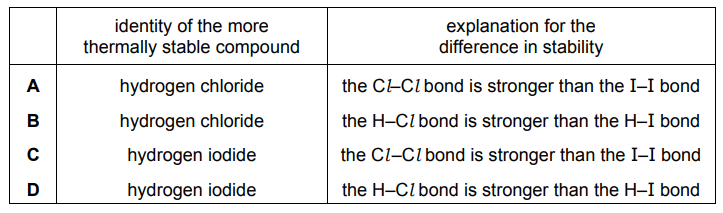
▶️Answer/Explanation
Ans:B
Question
Which atom has the same number of electrons as the hydroxide ion, \(OH^–\) ?
A F B Ne C Na D Mg
Answer/Explanation
Ans: B
Question
The volatility of the Group 17 elements, chlorine, bromine and iodine, decreases down the group.
What is responsible for this trend?
A bond length in the halogen molecule
B bond strength in the halogen molecule
C electronegativity of the halogen atom
D number of electrons in the halogen atom
Answer/Explanation
Answer: D
Question
Which row correctly describes the properties of the halogens as Group 17 is descended from chlorine to iodine?
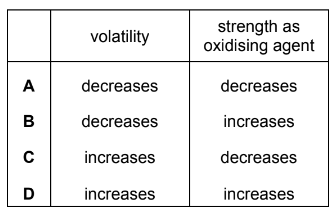
Answer/Explanation
Answer A
Question
The properties of chlorine, bromine and their compounds are compared.
Which property is smaller for chlorine than for bromine?
A bond strength of the hydrogen-halide bond
B first ionisation energy
C solubility of the silver halide in NH3(aq)
D strength of the van der Waals’ forces between molecules of the element
Answer/Explanation
Answer: D
Question
The volatility of the Group 17 elements, chlorine, bromine and iodine, decreases down the group.
What is responsible for this?
A bond length in the halogen molecule
B bond strength in the halogen molecule
C electronegativity of the halogen
D number of electrons in the halogen molecule
Answer/Explanation
Answer:
D
Question
The element astatine, At, is below iodine in Group 17 of the Periodic Table.
Which statements concerning At are likely to be correct?
1 It is a dark-coloured solid at room temperature.
2 It is a more powerful oxidising agent than iodine.
3 Its hydride is thermally stable.
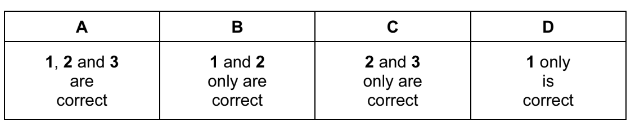
Answer/Explanation
Answer: D
Question
Why is the ionic radius of a chloride ion larger than the ionic radius of a sodium ion?
A A chloride ion has one more occupied electron shell than a sodium ion.
B Chlorine has a higher proton number than sodium.
C Ionic radius increases regularly across the third period.
D Sodium is a metal, chlorine is a non-metal.
Answer/Explanation
Answer: A
Question
Which statement about bromine is correct?
A Bromine is insoluble in non-polar solvents.
B Bromine vapour is more dense than air.
C Bromine will not vaporise significantly under normal conditions.
D Gaseous bromine is purple.
Answer/Explanation
Ans:B
Question
The element astatine, At, is below iodine in Group VII of the Periodic Table.
Which statements concerning At will be correct?
- It is a dark-coloured solid at room temperature.
- It is a more powerful oxidising agent than iodine.
- Its hydride is thermally stable.
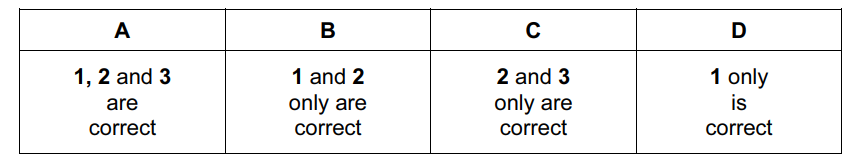
Answer/Explanation
Ans:
D
Question
What trend is observed on descending Group VII?
- The colours of the elements become lighter.
- The elements become more volatile.
- The hydrides of the elements become more thermally stable.
- The reactions of the elements with hydrogen become less vigorous.
Answer/Explanation
Ans:
D