Question
lodine is used in many inorganic and organic reactions.
(a) (i) State and explain the trend in volatility of the halogens, from chlorine to iodine.[2]
(ii) Explain why $\mathrm{HI}$ is the least thermally stable of $\mathrm{HCl}, \mathrm{HBr}$ and $\mathrm{HI}$.[1]
(iii) The table shows the electronegativity values for hydrogen, fluorine and iodine.
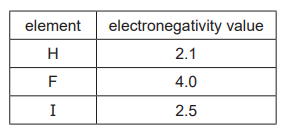
Explain, in terms of intermolecular forces, why HI has a lower boiling point than $\mathrm{HF}$.[2]
(iv) lodine reacts with hot concentrated aqueous sodium hydroxide in the same way as chlorine.
Write an equation for the reaction of iodine and hot aqueous sodium hydroxide. [1]
(b) lodoalkanes contain carbon-iodine bonds.
The simplest iodoalkane is $\mathrm{CH}_3 \mathrm{I}$.
(i) $\mathrm{CH}_3 \mathrm{I}$ can be made from methanol, $\mathrm{CH}_3 \mathrm{OH}$.
Identify a reagent that can convert $\mathrm{CH}_3 \mathrm{OH}$ to $\mathrm{CH}_3$ I.[1]
(ii) 1,2-diiodoethane, $\mathrm{CH}_2 \mathrm{ICH}_2 \mathrm{I}$, can be made by bubbling ethene into liquid iodine.
Fully name the type of mechanism shown in this reaction.[1]
(c) $\mathrm{J}$ reacts with $\mathrm{NaOH}$, forming different products dependent on the conditions used.
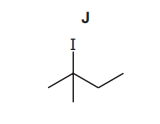
(i) Name J.
……………………………………………………………………………………………………………………… [1]
(ii) J reacts with NaOH(aq) to form K.
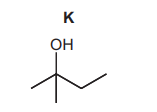
Fully name the mechanism of the reaction of J with NaOH(aq) to form K.
……………………………………………………………………………………………………………………… [1]
(iii) J reacts with NaOH dissolved in ethanol to form a mixture of two alkenes, L and M. Alkene L is shown.
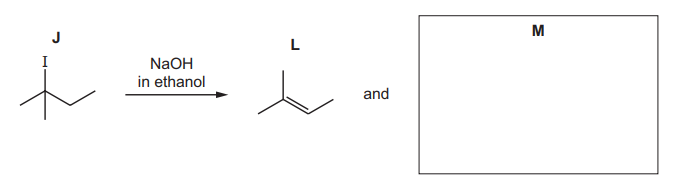
In the box provided, draw the structure of $\mathbf{M}$.
(iv) Explain why $\mathrm{L}$ does not show geometrical (cis-trans) isomerism.[1]
(v) L reacts with hot concentrated acidified $\mathrm{KMnO}_4(\mathrm{aq})$ to form propanone and one other organic product.
Identify the other organic product.[1]
(vi) Propanone reacts with excess alkaline aqueous iodine.
Complete and balance the equation for this reaction.
$
\mathrm{CH}_3 \mathrm{COCH}_3+\ldots . . \mathrm{I}_2+\ldots . \mathrm{OH}^{-} \longrightarrow \ldots . . \mathrm{CH}_3 \mathrm{COO}^{-}+\ldots . . \mathrm{H}_2 \mathrm{O}+\ldots . . \mathrm{I}^{-}+
$
(vii) State one observation that can be made in the reaction in (c)(vi).[1] [Total: 16]
▶️Answer/Explanation
Ans:
(a)(i) M1: (Volatility) decreases (down the group) 2
M2: more electrons so greater intermolecular forces / intermolecular attractions
OR
more electrons so greater VdW between molecules
(a)(ii) (HI has the) lowest bond enthalpy
(a)(iii) M1: HF has permanent dipole(-dipole forces) AND HI has ((only)) instantaneous dipole / induced dipole
(forces) / permanent dipole(-dipole forces)
M2: IMF’s in HI are weaker (than IMF’s in HF)
(a)(iv) $3 \mathrm{I}_2+6 \mathrm{NaOH} \rightarrow 5 \mathrm{NaI}+\mathrm{NaIO}_3+3 \mathrm{H}_2 \mathrm{O}$
(b)(i) $\mathrm{HI}(\mathrm{g}) / \mathrm{PI}_3 / \mathrm{P}$ and $\mathrm{I}_2$
(b)(ii) Electrophilic addition 1
(c)(i) 2(-)iodo(-)2(-)methylbutane
(c)(ii) Nucleophilic substitution / $\mathrm{S}_{\mathrm{N}}$
(c)(iii)
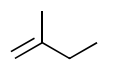
(c)(iv) ( L has) two identical / two methyl groups attached to one end / one carbon of the C=C / double bond
(c)(v) ethanoic acid $/ \mathrm{CH}_3 \mathrm{COOH}$
(c)(vi) $\mathrm{CH}_3 \mathrm{COCH}_3+3 \mathrm{I}_2+4 \mathrm{OH}^{-} \rightarrow(1) \mathrm{CH}_3 \mathrm{COO}^{-}+3 \mathrm{H}_2 \mathrm{O}+3 \mathrm{I}^{-}+\mathrm{CHI}_3$
M1: correctly balanced
M2: CHI3 product
(c)(vii) yellow ppt / yellow solid
Question:
The Group 17 elements, chlorine, bromine and iodine, are non-metals that show trends in their physical and chemical properties.
(a) Describe the trend in the colour of the Group 17 elements down the group.[1]
(b) The Group 17 elements can oxidise many metals to form halides.
(i) Describe the relative reactivity of the elements in Group 17 as oxidising agents.[1]
(ii) Chlorine reacts with hot tin metal to form tin(IV) chloride, $\mathrm{SnCl}_4$.
$\mathrm{SnCl}_4$ is a colourless liquid at room temperature that reacts vigorously with water to form an acidic solution.
Suggest the type of structure and bonding shown by $\mathrm{SnCl}_4$. Explain your answer.[2]
(c) The Group 17 elements form soluble halides with sodium.
(i) Describe what is seen when dilute $\mathrm{AgNO}_3(\mathrm{aq})$ is added to $\mathrm{NaBr}(\mathrm{aq})$ followed by aqueous ammonia. [2]
(ii) $\mathrm{NaCl}$ reacts with concentrated $\mathrm{H}_2 \mathrm{SO}_4$ to form $\mathrm{HCl}$ and $\mathrm{NaHSO}_4$.
Explain the difference between the reactions of concentrated $\mathrm{H}_2 \mathrm{SO}_4$ with $\mathrm{NaCl}$ and with NaI. Your answer should refer to the role of the sulfuric acid in each reaction.[3]
(d) The hydrogen halides are useful reagents in organic and inorganic reactions.
(i) Describe and explain the trend in the boiling points of the hydrogen halides, $\mathrm{HCl}, \mathrm{HBr}$ and HI.[2]
(ii) Describe and explain the trend in the thermal stabilities of the hydrogen halides, $\mathrm{HCl}, \mathrm{HBr}$ and $\mathrm{HI}$.
(e) Lucas’s reagent is a mixture of $\mathrm{HCl}$ and $\mathrm{ZnCl}_2$. Primary, secondary and tertiary alcohols can be distinguished by their reaction with Lucas’s reagent.
Alcohols react with the $\mathrm{HC} l$ in Lucas’s reagent to form halogenoalkanes.
$\mathrm{ZnCl}_2$ acts as a homogeneous catalyst for these reactions.
(i) Explain the meaning of the term homogeneous. [1]
(ii) Pentan-3-ol, $\mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{OH}) \mathrm{C}_2 \mathrm{H}_5$, reacts slowly with $\mathrm{HCl}$ to form a secondary halogenoalkane.
Complete the equation for this reaction using structural formulae.
$\mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{OH}) \mathrm{C}_2 \mathrm{H}_5+$
(iii) The fastest reaction shown by Lucas’s reagent is with a tertiary alcohol.
Draw the structure of the tertiary alcohol that is an isomer of pentan-3-ol.[1]
(iv) Tertiary alcohols tend to react with Lucas’s reagent using the same mechanism as in their reaction with $\mathrm{HCl}$.
Suggest the type of reaction shown by tertiary alcohols with Lucas’s reagent.[1][Total: 17]
▶️Answer/Explanation
Ans:
(a) darker / stronger / deeper down the group 1
(b)(i) weaker oxidising agents / (relative reactivity as oxidising agents) decreases down the group 1
(b)(ii) M1 (structure =) simple / molecular, because it has a low melting / boiling point
M2 (bonding =) covalent, because it is hydrolysed
(c)(i) M1 cream ppt / solid
M2 (ppt / solid) partially dissolves in (aqueous) ammonia
(c)(ii) M1 Acid behaviour of $\mathrm{H}_2 \mathrm{SO}_4$ $\mathrm{H}_2 \mathrm{SO}_4$ acts as an acid with $\mathrm{C} l^{-}$ $\mathrm{OR}$ acid / base reaction with $\mathrm{Cl}^{-}$
M2 Oxidising behaviour of $\mathrm{H}_2 \mathrm{SO}_4$ $\mathrm{H}_2 \mathrm{SO}_4$ acts as an oxidising agent with $\mathrm{I}^{-}$ $\mathrm{OR} \mathrm{H}_2 \mathrm{SO}_4$ does not oxidise $\mathrm{Cl}^{-}$
M3
Products formed (for iodide reaction) $\mathrm{I}_2 / \mathrm{S} / \mathrm{SO}_2 / \mathrm{H}_2 \mathrm{~S}$ is formed $\mathrm{OR}$ (for chloride reaction) (only) $\mathrm{HC} l$ is formed OR
Comparison of oxidising strength $\mathrm{H}_2 \mathrm{SO}_4$ not strong enough to / cannot oxidise $\mathrm{Cl}^{-}$(to $\mathrm{Cl}_2$ ) $\mathrm{OR} \mathrm{I}^{-}$more powerful reducing agent than $\mathrm{Cl}^{-}$
(d)(i) M1 increases (down the group) because of increasing VdW
M2 because of increasing number of electrons
(d)(ii) M1 less stable (down the group) / decreases
M2 lower H–Hal bond enthalpy / energy
(e)(i) in the same phase / state
(e)(ii) $\mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{OH}) \mathrm{C}_2 \mathrm{H}_5+\mathrm{HCl} \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{Cl}) \mathrm{C}_2 \mathrm{H}_5+\mathrm{H}_2 \mathrm{O}$
(e)(iii)
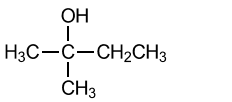
(e)(iv) substitution